Pyrene: Difference between revisions
CSV import |
CSV import |
||
| Line 24: | Line 24: | ||
{{stub}} | {{stub}} | ||
== Pyrene == | |||
<gallery> | |||
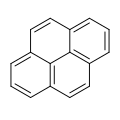
File:Pyrene.svg|Pyrene structure | |||
File:Br4Py_self-assembly_on_Au.jpg|Br4Py self-assembly on gold | |||
File:Br4Py_self-assembly_on_Au_2.jpg|Br4Py self-assembly on gold (second image) | |||
File:Pyrene-numbering.svg|Pyrene numbering | |||
</gallery> | |||
Latest revision as of 04:53, 18 February 2025
Pyrene is a polycyclic aromatic hydrocarbon (PAH) consisting of four fused benzene rings, resulting in a flat aromatic system. The chemical formula is C16H10. This yellow crystalline solid is the smallest peri-fused PAH (one where the rings are fused through more than one face). Pyrene forms during incomplete combustion of organic compounds.
Properties[edit]
Pyrene is a flat molecule that can intercalate into DNA, while it is not a particularly potent mutagen, it is quite toxic and teratogenic. Pyrene is also used as a probe to determine the polarity of a solvent. It also has a high quantum yield of fluorescence, and because of this it is used in biochemistry experiments as a fluorescent probe, particularly when studying lipids.
Occurrence[edit]
Pyrene and its derivatives are products of combustion and are produced by fossil fuel burning for heat and power, making them common air pollutants. Pyrene is not a major constituent of tar, but it is a component of soot, which is a byproduct of the combustion of diesel and other fuels.
Uses[edit]
Pyrene is used commercially to make dyes and dye precursors, and it is also used in research as a reference standard for the spectroscopic quantification of polycyclic aromatic hydrocarbons.
Health effects[edit]
Exposure to pyrene can cause skin irritation and inflammation. Long-term or repeated exposures can lead to more serious health effects, including cancer.