Testosterone phenylpropionate: Difference between revisions
No edit summary |
CSV import |
||
| Line 1: | Line 1: | ||
{{Short description|An overview of Testosterone phenylpropionate}} | |||
{{Drugbox | |||
| Verifiedfields = changed | |||
| verifiedrevid = 477002308 | |||
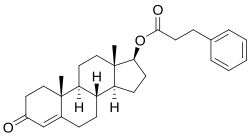
| IUPAC_name = 17β-Hydroxyandrost-4-en-3-one 3-phenylpropionate | |||
| image = Testosterone phenpropionate.svg | |||
| width = 200px | |||
}} | |||
'''Testosterone phenylpropionate''' is | '''Testosterone phenylpropionate''' is an [[androgen]] and [[anabolic steroid]] (AAS) and a [[testosterone]] [[ester]]. It was formerly used in [[medicine]] but is now mostly used in [[veterinary medicine]]. | ||
== | ==Pharmacology== | ||
Testosterone phenylpropionate is a synthetic | Testosterone phenylpropionate is a synthetic [[androgen]] and [[anabolic steroid]] and an [[androgen ester]]; specifically, it is the C17β [[phenylpropionate]] (phenylpropanoate) [[ester]] of [[testosterone]]. | ||
== | ===Mechanism of Action=== | ||
As an [[androgen]], testosterone phenylpropionate binds to the [[androgen receptor]], activating it and leading to the expression of specific genes. This results in the development and maintenance of [[male secondary sexual characteristics]] and the promotion of [[muscle growth]]. | |||
===Pharmacokinetics=== | |||
Testosterone phenylpropionate is administered via [[intramuscular injection]]. It has a relatively long half-life and duration of action due to the presence of the phenylpropionate ester, which slows the release of testosterone into the bloodstream. | |||
==Medical Uses== | |||
Testosterone phenylpropionate was used in the past for the treatment of [[male hypogonadism]], [[andropause]], and certain types of [[breast cancer]] in women. However, it has largely been replaced by other testosterone esters with more favorable pharmacokinetic profiles. | |||
== | ==Side Effects== | ||
Common side effects of testosterone phenylpropionate include [[acne]], [[oily skin]], [[hair loss]], and [[increased aggression]]. Long-term use can lead to [[cardiovascular disease]], [[liver damage]], and [[infertility]]. | |||
== | ==History== | ||
Testosterone phenylpropionate was first synthesized in the 1950s and was used in [[clinical practice]] for several decades. It was marketed under various brand names, including Testolent and Testoviron. | |||
== | ==Veterinary Use== | ||
In veterinary medicine, testosterone phenylpropionate is used to promote growth and improve feed efficiency in livestock. | |||
== | ==Also see== | ||
Testosterone | * [[Testosterone]] | ||
* [[Anabolic steroid]] | |||
[[ | * [[Androgen receptor]] | ||
[[ | * [[Testosterone enanthate]] | ||
[[Category:Androgens]] | * [[Testosterone cypionate]] | ||
[[Category: | |||
[[Category: | ==References== | ||
{{Reflist}} | |||
[[Category:Androgens and anabolic steroids]] | |||
[[Category:Testosterone esters]] | |||
[[Category:Veterinary drugs]] | |||
Latest revision as of 02:57, 11 December 2024
An overview of Testosterone phenylpropionate
| Testosterone phenylpropionate | |
|---|---|
| |
| INN | |
| Drug class | |
| Routes of administration | |
| Pregnancy category | |
| Bioavailability | |
| Metabolism | |
| Elimination half-life | |
| Excretion | |
| Legal status | |
| CAS Number | |
| PubChem | |
| DrugBank | |
| ChemSpider | |
| KEGG | |
Testosterone phenylpropionate is an androgen and anabolic steroid (AAS) and a testosterone ester. It was formerly used in medicine but is now mostly used in veterinary medicine.
Pharmacology[edit]
Testosterone phenylpropionate is a synthetic androgen and anabolic steroid and an androgen ester; specifically, it is the C17β phenylpropionate (phenylpropanoate) ester of testosterone.
Mechanism of Action[edit]
As an androgen, testosterone phenylpropionate binds to the androgen receptor, activating it and leading to the expression of specific genes. This results in the development and maintenance of male secondary sexual characteristics and the promotion of muscle growth.
Pharmacokinetics[edit]
Testosterone phenylpropionate is administered via intramuscular injection. It has a relatively long half-life and duration of action due to the presence of the phenylpropionate ester, which slows the release of testosterone into the bloodstream.
Medical Uses[edit]
Testosterone phenylpropionate was used in the past for the treatment of male hypogonadism, andropause, and certain types of breast cancer in women. However, it has largely been replaced by other testosterone esters with more favorable pharmacokinetic profiles.
Side Effects[edit]
Common side effects of testosterone phenylpropionate include acne, oily skin, hair loss, and increased aggression. Long-term use can lead to cardiovascular disease, liver damage, and infertility.
History[edit]
Testosterone phenylpropionate was first synthesized in the 1950s and was used in clinical practice for several decades. It was marketed under various brand names, including Testolent and Testoviron.
Veterinary Use[edit]
In veterinary medicine, testosterone phenylpropionate is used to promote growth and improve feed efficiency in livestock.
Also see[edit]
References[edit]
<references group="" responsive="1"></references>