Uranium
Uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. Uranium is weakly radioactive because all its isotopes are unstable, with half-lives varying between 159,200 years and 4.5 billion years.
Properties[edit]
Uranium is a dense metal that has the highest atomic weight of the naturally occurring elements. It is approximately 70% denser than lead and slightly less dense than gold. Uranium metal reacts with almost all non-metal elements and their compounds, with reactivity increasing with temperature.
Isotopes[edit]
Uranium has several isotopes, the most common of which are Uranium-238 and Uranium-235. Uranium-238 is the most abundant isotope, making up about 99.3% of natural uranium, while Uranium-235 accounts for about 0.7%.
Uranium-238[edit]
Uranium-238 is not fissile, meaning it cannot sustain a nuclear chain reaction. However, it is fertile, meaning it can be converted into a fissile material, such as Plutonium-239, in a nuclear reactor.
Uranium-235[edit]
Uranium-235 is the only naturally occurring fissile isotope, which means it can sustain a nuclear chain reaction. This property makes it critical for both nuclear power generation and nuclear weapons.
Uses[edit]
Uranium is primarily used as fuel in nuclear reactors to generate electricity. It is also used in the manufacture of nuclear weapons. Depleted uranium, which is uranium with a lower content of Uranium-235 than natural uranium, is used in armor-piercing ammunition and in radiation shielding.
Health Effects[edit]
Exposure to uranium can lead to both chemical and radiological health effects. Chemically, uranium is a heavy metal and can cause kidney damage. Radiologically, uranium is a source of ionizing radiation, which can increase the risk of cancer.
Chemical Toxicity[edit]
Uranium can be toxic to the kidneys if ingested or inhaled in large amounts. The chemical toxicity of uranium is more significant than its radiological effects at low exposure levels.
Radiological Effects[edit]
Uranium emits alpha particles, which are not penetrating and can be stopped by a sheet of paper or the outer layer of human skin. However, if uranium is ingested or inhaled, it can irradiate internal organs.
Environmental Impact[edit]
Uranium mining and milling can have significant environmental impacts, including habitat destruction and contamination of water sources. The disposal of uranium mill tailings, which contain radioactive decay products, is a major environmental concern.
See Also[edit]
External Links[edit]
- [International Atomic Energy Agency](https://www.iaea.org/)
- [World Nuclear Association](https://www.world-nuclear.org/)
Uranium[edit]
-
Nuclear fission
-
30mm DU slug
-
U glass with black light
-
Uranium ceramic
-
Vacuum capacitor with uranium glass
-
Becquerel plate
-
Uranium cubes
-
Atomic cloud over Hiroshima
-
First four nuclear lit bulbs
-
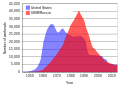
US and USSR nuclear stockpiles
-
Pichblende
Ad. Transform your life with W8MD's Budget GLP-1 injections from $75


W8MD offers a medical weight loss program to lose weight in Philadelphia. Our physician-supervised medical weight loss provides:
- Weight loss injections in NYC (generic and brand names):
- Zepbound / Mounjaro, Wegovy / Ozempic, Saxenda
- Most insurances accepted or discounted self-pay rates. We will obtain insurance prior authorizations if needed.
- Generic GLP1 weight loss injections from $75 for the starting dose.
- Also offer prescription weight loss medications including Phentermine, Qsymia, Diethylpropion, Contrave etc.
NYC weight loss doctor appointmentsNYC weight loss doctor appointments
Start your NYC weight loss journey today at our NYC medical weight loss and Philadelphia medical weight loss clinics.
- Call 718-946-5500 to lose weight in NYC or for medical weight loss in Philadelphia 215-676-2334.
- Tags:NYC medical weight loss, Philadelphia lose weight Zepbound NYC, Budget GLP1 weight loss injections, Wegovy Philadelphia, Wegovy NYC, Philadelphia medical weight loss, Brookly weight loss and Wegovy NYC
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian