Sodium trimetaphosphate

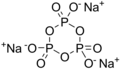
Sodium trimetaphosphate (IUPAC name: sodium trioxido-1κO^4,1κO^5,1κO^6-triphosphate), is a chemical compound with the formula Na3P3O9. It is one of the sodium phosphates used in a variety of industrial and food applications. Sodium trimetaphosphate is a salt that consists of sodium ions and trimetaphosphate anions. It appears as a white, crystalline powder that is soluble in water, producing an alkaline solution.
Production[edit]
Sodium trimetaphosphate is produced by heating monosodium phosphate, NaH2PO4, or disodium phosphate, Na2HPO4, to high temperatures, which induces a condensation reaction that results in the formation of the trimetaphosphate ion, P3O9^3−.
Applications[edit]
Food Industry[edit]
In the food industry, sodium trimetaphosphate is utilized as an emulsifier, to improve the texture of processed foods, and as a sequestrant, helping to bind divalent metal ions. It is also used in the production of certain types of processed cheese, seafood products, and meat processing, where it acts as a water retention agent.
Industrial Uses[edit]
Beyond its applications in food, sodium trimetaphosphate finds use in various industrial processes. It serves as a dispersing agent in ceramics, detergents, and paints. Additionally, it is employed in water treatment as a sequestrant to remove hardness from water and in the oil industry for drilling fluids.
Health and Safety[edit]
The safety of sodium trimetaphosphate is overseen by food and health regulatory bodies, such as the U.S. Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA), which evaluate its use in food and other products. While generally recognized as safe when used within prescribed limits, excessive intake of phosphates, including sodium trimetaphosphate, can lead to health issues, particularly in individuals with kidney problems or those at risk of developing kidney disease.
Environmental Impact[edit]
The environmental impact of sodium trimetaphosphate is similar to that of other phosphate salts. Its use in detergents and other applications can contribute to eutrophication in water bodies, leading to algal blooms and subsequent depletion of oxygen levels in aquatic environments. Therefore, its discharge into the environment is regulated in many jurisdictions.
See Also[edit]
-
Sodium trimetaphosphate
-
Sodium trimetaphosphate
Ad. Transform your life with W8MD's Budget GLP-1 injections from $49.99


W8MD offers a medical weight loss program to lose weight in Philadelphia. Our physician-supervised medical weight loss provides:
- Weight loss injections in NYC (generic and brand names):
- Zepbound / Mounjaro, Wegovy / Ozempic, Saxenda
- Most insurances accepted or discounted self-pay rates. We will obtain insurance prior authorizations if needed.
- Generic GLP1 weight loss injections from $49.99 for the starting dose of Semaglutide and $65.00 for Tirzepatide.
- Also offer prescription weight loss medications including Phentermine, Qsymia, Diethylpropion, Contrave etc.
NYC weight loss doctor appointmentsNYC weight loss doctor appointments
Start your NYC weight loss journey today at our NYC medical weight loss and Philadelphia medical weight loss clinics.
- Call 718-946-5500 to lose weight in NYC or for medical weight loss in Philadelphia 215-676-2334.
- Tags:NYC medical weight loss, Philadelphia lose weight Zepbound NYC, Budget GLP1 weight loss injections, Wegovy Philadelphia, Wegovy NYC, Philadelphia medical weight loss, Brookly weight loss and Wegovy NYC
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian