Nylon
Nylon[edit]

Nylon is a generic designation for a family of synthetic polymers known as polyamides. It was first produced on February 28, 1935, by Wallace Carothers at DuPont, a major American chemical company. Nylon is a thermoplastic silky material that can be melt-processed into fibers, films, or shapes. It is one of the most widely used polymers.
History[edit]

The development of nylon began in the early 1930s when Wallace Carothers, a chemist at DuPont, was tasked with creating a synthetic fiber that could replace silk. Carothers and his team successfully synthesized the first nylon polymer in 1935. The first commercial use of nylon was in a nylon-bristled toothbrush in 1938, followed by women's stockings in 1940, which became immensely popular.
Chemical Structure[edit]
Nylon is a polyamide, which means it contains repeating units linked by amide bonds. The most common types of nylon are Nylon 6 and Nylon 6,6. Nylon 6 is made from a single type of monomer, caprolactam, while Nylon 6,6 is made from two monomers, hexamethylenediamine and adipic acid. The polymerization process involves a condensation reaction, where water is released as a byproduct.

Properties[edit]
Nylon is known for its high tensile strength, elasticity, and resistance to abrasion and chemicals. It is also resistant to heat and can be dyed easily. These properties make it suitable for a wide range of applications, from clothing to industrial uses.
Applications[edit]

Nylon is used in a variety of applications, including:
- Textiles and Fabrics: Nylon is used in the production of hosiery, swimwear, activewear, and other clothing items due to its elasticity and strength.
- Industrial Uses: It is used in the manufacture of ropes, conveyor belts, and automotive parts.
- Consumer Goods: Nylon is used in toothbrush bristles, fishing lines, and guitar strings.
Environmental Impact[edit]
The production of nylon is energy-intensive and involves the use of petrochemicals, which contribute to environmental pollution. However, efforts are being made to recycle nylon products and develop more sustainable production methods.

Microstructure[edit]
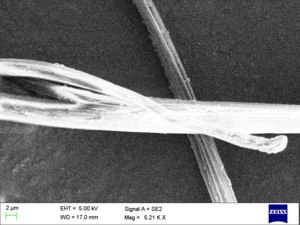
Nylon fibers have a crystalline structure that contributes to their strength and durability. The hydrogen bonds between the polymer chains enhance the material's mechanical properties.

Related Pages[edit]
Ad. Transform your life with W8MD's Budget GLP-1 injections from $75


W8MD offers a medical weight loss program to lose weight in Philadelphia. Our physician-supervised medical weight loss provides:
- Weight loss injections in NYC (generic and brand names):
- Zepbound / Mounjaro, Wegovy / Ozempic, Saxenda
- Most insurances accepted or discounted self-pay rates. We will obtain insurance prior authorizations if needed.
- Generic GLP1 weight loss injections from $75 for the starting dose.
- Also offer prescription weight loss medications including Phentermine, Qsymia, Diethylpropion, Contrave etc.
NYC weight loss doctor appointmentsNYC weight loss doctor appointments
Start your NYC weight loss journey today at our NYC medical weight loss and Philadelphia medical weight loss clinics.
- Call 718-946-5500 to lose weight in NYC or for medical weight loss in Philadelphia 215-676-2334.
- Tags:NYC medical weight loss, Philadelphia lose weight Zepbound NYC, Budget GLP1 weight loss injections, Wegovy Philadelphia, Wegovy NYC, Philadelphia medical weight loss, Brookly weight loss and Wegovy NYC
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian