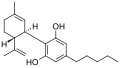
Nabiximols
Nabiximols is a cannabinoid-based medicine derived from the cannabis plant. It is a complex botanical mixture containing the principal cannabinoids tetrahydrocannabinol (THC) and cannabidiol (CBD), as well as other minor cannabinoids and non-cannabinoid components. Nabiximols is marketed under the brand name Sativex and is primarily used for the treatment of spasticity in multiple sclerosis (MS) patients, although it has also been investigated for its potential in treating pain, overactive bladder, and other medical conditions.
Composition and Production[edit]
Nabiximols is produced from specifically bred cannabis plants and is formulated as an oromucosal spray, allowing for administration through the oral mucosa. Each spray delivers a fixed dose of THC and CBD, along with trace amounts of other cannabinoids. The precise composition and the ratio of THC to CBD can vary depending on the product and manufacturer, but it generally aims to maintain a balanced profile to mitigate the psychoactive effects of THC while leveraging the therapeutic benefits of both cannabinoids.
Mechanism of Action[edit]
The therapeutic effects of nabiximols are believed to result from its action on the endocannabinoid system, a complex network of cannabinoid receptors (primarily CB1 and CB2 receptors) and endogenous cannabinoids (endocannabinoids) that play a role in various physiological processes. THC and CBD interact with these receptors, though in different ways. THC is a partial agonist of both CB1 and CB2 receptors, which can help reduce pain and spasticity, while CBD has a more complex mechanism, including antagonistic effects on certain receptors that may contribute to its anti-inflammatory and analgesic properties.
Clinical Uses[edit]
Nabiximols is approved in several countries for the treatment of spasticity due to multiple sclerosis that has not responded adequately to other medications. Patients report improvements in symptoms such as muscle stiffness, pain, and sleep disturbances. The drug is also being investigated for its potential in treating other conditions, including chronic pain, cancer pain, and neuropathic pain, although its use in these areas is not yet widely approved.
Side Effects and Contraindications[edit]
Common side effects of nabiximols include dizziness, fatigue, and oral discomfort or pain. These effects are generally mild to moderate and tend to decrease with continued use. Nabiximols is contraindicated in patients with a history of serious psychiatric disorders, particularly schizophrenia, due to the potential for exacerbation of symptoms. It is also not recommended for use in pregnant or breastfeeding women due to the lack of comprehensive safety data.
Regulatory Status[edit]
The regulatory status of nabiximols varies by country. In the United Kingdom, it was the first cannabis-based medicine to be licensed when it received approval for MS spasticity. It has also been approved in several other European countries, Canada, and Australia for similar uses. In the United States, nabiximols is classified as a Schedule I controlled substance, and its approval and availability are subject to ongoing clinical trials and regulatory review.
Future Directions[edit]
Research into nabiximols and other cannabinoid-based therapies is ongoing, with studies exploring their potential in treating a wider range of conditions, including epilepsy, glaucoma, and anxiety disorders. As the medical community gains a better understanding of the endocannabinoid system and the complex pharmacology of cannabis-derived compounds, the therapeutic applications of nabiximols may expand.
Nabiximols[edit]
-
THC
-
Cannabidiol
-
Sativex - Canadian box front
Ad. Transform your life with W8MD's Budget GLP-1 injections from $75


W8MD offers a medical weight loss program to lose weight in Philadelphia. Our physician-supervised medical weight loss provides:
- Weight loss injections in NYC (generic and brand names):
- Zepbound / Mounjaro, Wegovy / Ozempic, Saxenda
- Most insurances accepted or discounted self-pay rates. We will obtain insurance prior authorizations if needed.
- Generic GLP1 weight loss injections from $75 for the starting dose.
- Also offer prescription weight loss medications including Phentermine, Qsymia, Diethylpropion, Contrave etc.
NYC weight loss doctor appointmentsNYC weight loss doctor appointments
Start your NYC weight loss journey today at our NYC medical weight loss and Philadelphia medical weight loss clinics.
- Call 718-946-5500 to lose weight in NYC or for medical weight loss in Philadelphia 215-676-2334.
- Tags:NYC medical weight loss, Philadelphia lose weight Zepbound NYC, Budget GLP1 weight loss injections, Wegovy Philadelphia, Wegovy NYC, Philadelphia medical weight loss, Brookly weight loss and Wegovy NYC
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian