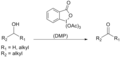
Dess–Martin periodinane
Dess–Martin periodinane (DMP) is an organic compound used primarily as an oxidizing agent in organic synthesis. Developed by Daniel Benjamin Dess and James Cullen Martin in 1983, DMP has become a popular choice for selectively oxidizing primary alcohols to aldehydes and secondary alcohols to ketones with minimal overoxidation to carboxylic acids. Its chemical formula is C₁₃H₁₅IO₇.
Properties[edit]
Dess–Martin periodinane is characterized by its stability and mildness as an oxidizing agent. Unlike other oxidizing agents, DMP operates under relatively mild conditions, thus preserving sensitive functional groups in the substrate. It is a white to off-white crystalline solid, soluble in organic solvents such as dichloromethane, chloroform, and acetonitrile.
Synthesis[edit]
The synthesis of Dess–Martin periodinane involves the reaction of Iodoxybenzoic acid (IBX) with 1,1,1-triisopropylbenzene under acidic conditions to yield DMP. This process highlights the transformation of IBX, a potent oxidizing agent in its own right, into a more selective and user-friendly reagent.
Mechanism[edit]
The mechanism of action for DMP involves the formation of an iodonium ion intermediate, which facilitates the transfer of an oxygen atom to the alcohol substrate. This results in the formation of the desired ketone or aldehyde product and the reduction of DMP to iodylbenzene diacetate, which can be easily separated from the reaction mixture.
Applications[edit]
Dess–Martin periodinane has found widespread use in organic synthesis, including the synthesis of complex natural products and pharmaceuticals. Its ability to selectively oxidize alcohols without affecting other functional groups makes it an invaluable tool in the chemist's toolkit. DMP is particularly useful in the final steps of synthesis, where the selective oxidation of a primary or secondary alcohol can be crucial to obtaining the desired product.
Safety and Handling[edit]
While Dess–Martin periodinane is considered less hazardous than many other oxidizing agents, it should still be handled with care. Precautions should be taken to avoid inhalation and contact with skin or eyes. Use in a well-ventilated area and personal protective equipment such as gloves and goggles are recommended.
Environmental Considerations[edit]
The use of DMP in organic synthesis poses minimal environmental risk when used and disposed of properly. However, as with all chemical reagents, responsible use and disposal practices should be followed to minimize any potential environmental impact.
See Also[edit]
Dess–Martin periodinane[edit]
-
Dess–Martin periodinane
-
Dess–Martin periodinane 3D model
-
Reaction scheme
-
Preparation of DMP
-
Dess–Martin synthesis
-
Oxidation by Dess–Martin periodinane
-
Water addition
-
Dess–Martin periodinane oxidation
-
T-butyl DMP
Ad. Transform your life with W8MD's Budget GLP-1 injections from $75


W8MD offers a medical weight loss program to lose weight in Philadelphia. Our physician-supervised medical weight loss provides:
- Weight loss injections in NYC (generic and brand names):
- Zepbound / Mounjaro, Wegovy / Ozempic, Saxenda
- Most insurances accepted or discounted self-pay rates. We will obtain insurance prior authorizations if needed.
- Generic GLP1 weight loss injections from $75 for the starting dose.
- Also offer prescription weight loss medications including Phentermine, Qsymia, Diethylpropion, Contrave etc.
NYC weight loss doctor appointmentsNYC weight loss doctor appointments
Start your NYC weight loss journey today at our NYC medical weight loss and Philadelphia medical weight loss clinics.
- Call 718-946-5500 to lose weight in NYC or for medical weight loss in Philadelphia 215-676-2334.
- Tags:NYC medical weight loss, Philadelphia lose weight Zepbound NYC, Budget GLP1 weight loss injections, Wegovy Philadelphia, Wegovy NYC, Philadelphia medical weight loss, Brookly weight loss and Wegovy NYC
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian