Benzotriazole


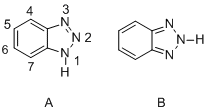



Benzotriazole (BTA) is an organic compound that is a heterocyclic compound with the chemical formula C_6H_5N_3. It consists of a benzene ring fused to a triazole ring. Benzotriazole is a colorless solid that is soluble in a range of organic solvents. It is widely used in industry, particularly in the fields of photography, pharmaceuticals, agrochemicals, and as a corrosion inhibitor in cooling systems and in the atmosphere.
Properties[edit]
Benzotriazole has a melting point of 98-100 °C and a boiling point of 204 °C (399 °F) at 13 mmHg. It is slightly soluble in water, but highly soluble in most organic solvents such as alcohols, acetone, and benzene. Its solubility in water and its high boiling point make it useful in a wide range of applications, especially as a corrosion inhibitor.
Applications[edit]
Corrosion Inhibition[edit]
One of the primary uses of benzotriazole is as a corrosion inhibitor, particularly for copper and its alloys. It forms a complex with copper ions, creating a protective layer on the metal's surface that significantly reduces corrosion. This property is exploited in cooling systems, including those used in automotive and industrial applications, and in the protection of outdoor copper and bronze sculptures and monuments.
Photography[edit]
In photography, benzotriazole is used as an antifoggant and restrainer. It helps in controlling the development of photographic films and papers, ensuring clearer images and greater detail.
Pharmaceuticals[edit]
In the pharmaceutical industry, benzotriazole derivatives are explored for their potential therapeutic properties. They have been studied for their antiviral, antibacterial, and anticancer activities, among others.
Agrochemicals[edit]
Benzotriazole also finds application in the production of agrochemicals. It is used in the synthesis of herbicides, fungicides, and plant growth regulators, contributing to the protection of crops from pests and diseases.
Environmental Impact[edit]
While benzotriazole is valuable in various industrial applications, its environmental impact is a growing concern. It is persistent in the environment and has been detected in water bodies, raising questions about its effects on aquatic life and potential for bioaccumulation. Research is ongoing to understand its environmental fate and to develop strategies for its management and removal from contaminated sites.
Safety[edit]
Benzotriazole is generally considered to be of low toxicity, but it can cause irritation to the skin, eyes, and respiratory tract upon exposure. Proper handling and protective equipment are recommended when working with this chemical.
Ad. Transform your life with W8MD's Budget GLP-1 injections from $75


W8MD offers a medical weight loss program to lose weight in Philadelphia. Our physician-supervised medical weight loss provides:
- Weight loss injections in NYC (generic and brand names):
- Zepbound / Mounjaro, Wegovy / Ozempic, Saxenda
- Most insurances accepted or discounted self-pay rates. We will obtain insurance prior authorizations if needed.
- Generic GLP1 weight loss injections from $75 for the starting dose.
- Also offer prescription weight loss medications including Phentermine, Qsymia, Diethylpropion, Contrave etc.
NYC weight loss doctor appointmentsNYC weight loss doctor appointments
Start your NYC weight loss journey today at our NYC medical weight loss and Philadelphia medical weight loss clinics.
- Call 718-946-5500 to lose weight in NYC or for medical weight loss in Philadelphia 215-676-2334.
- Tags:NYC medical weight loss, Philadelphia lose weight Zepbound NYC, Budget GLP1 weight loss injections, Wegovy Philadelphia, Wegovy NYC, Philadelphia medical weight loss, Brookly weight loss and Wegovy NYC
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian
