Hammerhead ribozyme: Difference between revisions
CSV import |
CSV import |
||
| Line 22: | Line 22: | ||
{{biology-stub}} | {{biology-stub}} | ||
<gallery> | |||
File:Hammerhead ribozyme ribbons.png|Hammerhead ribozyme ribbons | |||
File:RF02275-rscape.svg|RF02275 rscape | |||
File:Hammerhead ribozyme electron transfer mechanism.svg|Hammerhead ribozyme electron transfer mechanism | |||
File:Minimal and full length hammerhead sequences.png|Minimal and full length hammerhead sequences | |||
File:RF00163-rscape.svg|RF00163 rscape | |||
File:RF00008-rscape.svg|RF00008 rscape | |||
File:Minimal hammerhead ribozyme structure.png|Minimal hammerhead ribozyme structure | |||
File:Full length hammerhead ribozyme.png|Full length hammerhead ribozyme | |||
File:Acid base hammerhead ribozyme.png|Acid base hammerhead ribozyme | |||
File:Hammerhead ribozyme mechanism.png|Hammerhead ribozyme mechanism | |||
</gallery> | |||
Latest revision as of 05:39, 3 March 2025
Hammerhead ribozyme is a type of RNA molecule that belongs to a class of catalytic RNA or ribozymes, which are capable of catalyzing specific biochemical reactions, similar to the action of protein enzymes. The hammerhead ribozyme is notable for its self-cleaving activity, making it an important subject in the study of molecular biology, genetics, and biotechnology. This ribozyme was first discovered in the 1980s within the virusoid RNA of plant pathogens but has since been found in all domains of life, indicating a widespread evolutionary significance.
Structure[edit]
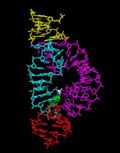
The hammerhead ribozyme consists of a conserved core of about 11-12 nucleotides surrounded by three helical arms forming a Y-shaped structure. This core includes several critical bases that are essential for the catalytic activity of the ribozyme. The structure is stabilized by both Watson-Crick base pairing and non-Watson-Crick interactions, which are crucial for its catalytic function. The three-dimensional structure of the hammerhead ribozyme has been elucidated through X-ray crystallography and NMR spectroscopy, revealing the detailed interactions within the RNA molecule that enable its catalytic activity.
Mechanism[edit]
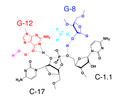
The catalytic mechanism of the hammerhead ribozyme involves the cleavage of a specific phosphodiester bond within an RNA substrate. This reaction is facilitated by the formation of a complex between the ribozyme and its RNA substrate, aligning the scissile phosphate for nucleophilic attack. The cleavage reaction results in the breakage of the RNA backbone, leading to two separate RNA fragments. The hammerhead ribozyme mechanism is a model for understanding RNA catalysis and has implications for the design of RNA-based therapeutic agents.
Biological Significance[edit]
The discovery of the hammerhead ribozyme contributed significantly to the understanding of the catalytic potential of RNA molecules, supporting the RNA world hypothesis, which suggests that early life forms may have relied on RNA for both genetic information storage and catalysis. In nature, hammerhead ribozymes are thought to regulate gene expression and participate in the replication cycle of certain viruses and virusoids.
Applications[edit]
Due to its specific cleavage activity, the hammerhead ribozyme has been explored as a tool for gene therapy and the control of viral infections. By designing hammerhead ribozymes that target specific RNA sequences, researchers can potentially inhibit the expression of harmful genes or viral genomes. This approach has been investigated for the treatment of various diseases, including cancer, HIV/AIDS, and genetic disorders.
Research and Development[edit]
Ongoing research aims to improve the stability and specificity of hammerhead ribozymes for therapeutic applications. This includes modifications to the ribozyme structure to enhance its resistance to degradation in biological systems and to increase its affinity for target RNA molecules. Advances in the field of synthetic biology and nanotechnology are also enabling the development of novel delivery systems for ribozyme-based therapies.

-
Hammerhead ribozyme ribbons
-
RF02275 rscape
-
Hammerhead ribozyme electron transfer mechanism
-
Minimal and full length hammerhead sequences
-
RF00163 rscape
-
RF00008 rscape
-
Minimal hammerhead ribozyme structure
-
Full length hammerhead ribozyme
-
Acid base hammerhead ribozyme
-
Hammerhead ribozyme mechanism