Ethyl cinnamate: Difference between revisions
CSV import |
CSV import |
||
| Line 24: | Line 24: | ||
{{Chem-stub}} | {{Chem-stub}} | ||
{{No image}} | {{No image}} | ||
<gallery> | |||
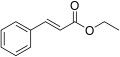
File:Ethyl-cinnamate.svg|Ethyl-cinnamate.svg | |||
File:Ethyl_cinnamate_3D_spacefill.png|Ethyl_cinnamate_3D_spacefill.png | |||
</gallery> | |||
Revision as of 21:37, 20 February 2025

Ethyl cinnamate is an organic compound that belongs to the ester class of chemicals, specifically an ester of cinnamic acid and ethanol. It is known for its sweet, balsamic aroma, which makes it a popular additive in the flavor and fragrance industries. Ethyl cinnamate is found naturally in various plants and is also synthesized for commercial use.
Properties
Ethyl cinnamate is a colorless liquid at room temperature with a sweet, fruity, and spicy aroma. Its chemical formula is C11H12O2, and it has a molecular weight of 176.21 g/mol. This compound is slightly soluble in water but highly soluble in organic solvents such as ethanol, diethyl ether, and benzene. The boiling point of ethyl cinnamate is approximately 271°C.
Synthesis
Ethyl cinnamate can be synthesized through an esterification reaction between cinnamic acid and ethanol. This reaction typically requires the presence of an acid catalyst, such as sulfuric acid, to proceed efficiently. The process involves heating the reactants together with the catalyst, which facilitates the formation of ethyl cinnamate and water as by-products.
Applications
Ethyl cinnamate is widely used in the flavor and fragrance industry due to its pleasant aroma. It is a component of various food flavorings, perfumes, and cosmetic products. In the food industry, it is used to impart a sweet, balsamic flavor to confectioneries, beverages, and baked goods. In perfumery, it is valued for its spicy, fruity notes, contributing to the complexity of fragrances.
Safety
As with many chemical compounds, the safety of ethyl cinnamate depends on its concentration and the manner of exposure. It is generally regarded as safe when used in small quantities as a flavoring agent or fragrance. However, like many esters, it can cause irritation to the skin, eyes, and respiratory system upon prolonged or excessive exposure. Proper handling and adherence to safety guidelines are essential when working with ethyl cinnamate in industrial settings.
Environmental Impact
The environmental impact of ethyl cinnamate is considered to be low. It is biodegradable and does not accumulate in the environment. However, as with all chemical manufacturing processes, the production of ethyl cinnamate can have environmental implications, including the use of resources and generation of waste. It is important for industries to follow environmental regulations and adopt sustainable practices in the production and disposal of ethyl cinnamate.
-
Ethyl-cinnamate.svg
-
Ethyl_cinnamate_3D_spacefill.png