Lactone: Difference between revisions
CSV import |
CSV import |
||
| Line 30: | Line 30: | ||
{{stub}} | {{stub}} | ||
== Lactone == | |||
<gallery> | |||
File:Acetolactone-2D-skeletal.png|Acetolactone | |||
File:Propiolactone.png|Propiolactone | |||
File:GBL_chemical_structure.svg|Gamma-Butyrolactone (GBL) | |||
File:Caprolactone.png|Caprolactone | |||
File:Glucono-delta-lactone-2D-skeletal.png|Glucono delta-lactone | |||
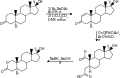
File:Oxandrolones_synthesis.svg|Oxandrolone synthesis | |||
File:Iodolactonization.svg|Iodolactonization | |||
File:γ-Lactones_synthesis.svg|Gamma-Lactones synthesis | |||
File:Polylactide_synthesis_v.1.png|Polylactide synthesis | |||
</gallery> | |||
Latest revision as of 11:20, 18 February 2025
Lactone is a cyclic ester that results from the dehydration of a hydroxy acid. They are involved in a wide range of biological processes, including the production of certain antibiotics and cancer drugs. Lactones are classified into small and large, depending on the size of the lactone ring.
Structure and Classification[edit]
Lactones are characterized by a carbonyl group (C=O) and a hydroxyl group (OH) in the same molecule. The number of carbon atoms in the lactone ring determines its size and classification. Small lactones, such as butyrolactone and valerolactone, have a three or four-membered lactone ring. Large lactones, such as macrolides, have a 12 to 20-membered lactone ring.
Synthesis[edit]
Lactones are synthesized through the dehydration of hydroxy acids or the intramolecular esterification of a carboxylic acid. The process involves the removal of a water molecule or the addition of a catalyzing agent such as sulfuric acid.
Applications[edit]
Lactones have a wide range of applications in the pharmaceutical and food industries. They are used in the synthesis of certain antibiotics, such as erythromycin and clindamycin, and cancer drugs, such as doxorubicin and etoposide. In the food industry, lactones are used as flavoring agents due to their sweet or fruity taste.
See Also[edit]
References[edit]
<references />