Drug-eluting stent: Difference between revisions
CSV import |
CSV import |
||
| Line 34: | Line 34: | ||
{{Medicine-stub}} | {{Medicine-stub}} | ||
<gallery> | |||
File:Taxus_stent_FDA.jpg|Taxus stent approved by FDA | |||
File:Blausen_0034_Angioplasty_Stent_01.png|Illustration of angioplasty with stent | |||
File:Coronary_arteries.svg|Diagram of coronary arteries | |||
File:PTCA_stent_NIH.gif|PTCA stent procedure | |||
File:Drug-eluting_stent.jpg|Drug-eluting stent | |||
</gallery> | |||
Latest revision as of 04:50, 18 February 2025
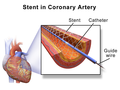
Drug-eluting stent (DES) is a percutaneous coronary intervention device used in the treatment of coronary artery disease (CAD). It consists of a metal stent that is coated with a pharmacologic agent (drug) that is released (eluted) slowly to inhibit cell proliferation. This prevents fibrosis that, together with clots (thrombi), could otherwise block the stented artery, a process known as in-stent restenosis. The development of DES was a major advancement in the treatment of coronary artery disease, particularly in reducing the incidence of in-stent restenosis when compared to bare-metal stents (BMS).
History[edit]
The first drug-eluting stent was approved by the U.S. Food and Drug Administration (FDA) in 2003. This innovation was built upon the earlier development of bare-metal stents, which provided a scaffold to keep the artery open but did not address the issue of restenosis caused by neointimal hyperplasia.
Mechanism of Action[edit]
Drug-eluting stents release a drug into the artery wall that inhibits the growth of scar tissue, reducing the risk of restenosis. The drugs used are typically immunosuppressive or antineoplastic agents, such as sirolimus, paclitaxel, everolimus, or zotarolimus. These drugs target the cells responsible for inflammation and proliferation within the artery wall after stent implantation.
Types[edit]
There are several types of drug-eluting stents, categorized based on the type of drug used and the polymer coating that controls the release of the drug. First-generation DES include sirolimus-eluting and paclitaxel-eluting stents, while newer generations use drugs like everolimus and zotarolimus, which may offer improved outcomes in terms of reducing restenosis and thrombosis rates.
Indications[edit]
Drug-eluting stents are indicated for use in patients with coronary artery disease who are undergoing angioplasty. They are particularly beneficial for patients at high risk of restenosis, such as those with diabetes mellitus, long lesions, or small diameter vessels.
Complications[edit]
While drug-eluting stents significantly reduce the risk of restenosis, they are not without risks. Potential complications include stent thrombosis, a rare but serious event that can lead to myocardial infarction or death. Other risks include bleeding due to the prolonged use of antiplatelet therapy, hypersensitivity reactions to the stent's components, and late restenosis.
Clinical Trials[edit]
Numerous clinical trials have demonstrated the efficacy and safety of drug-eluting stents. The RAVEL, SIRIUS, TAXUS, and HORIZONS-AMI trials are among the most notable, each showing a significant reduction in restenosis rates and major adverse cardiac events (MACE) when compared to bare-metal stents.
Future Directions[edit]
Research continues to focus on improving the design and pharmacology of drug-eluting stents to reduce the incidence of stent thrombosis, enhance biocompatibility, and potentially eliminate the need for prolonged antiplatelet therapy. Bioresorbable stents, which fully dissolve after serving their purpose, represent one area of ongoing investigation.
See Also[edit]
- Percutaneous coronary intervention
- Coronary artery disease
- Bare-metal stent
- Restenosis
- Stent thrombosis
-
Taxus stent approved by FDA
-
Illustration of angioplasty with stent
-
Diagram of coronary arteries
-
PTCA stent procedure
-
Drug-eluting stent