Phthalic acid: Difference between revisions
CSV import |
CSV import |
||
| Line 28: | Line 28: | ||
[[Category:Endocrine disruptors]] | [[Category:Endocrine disruptors]] | ||
{{Chem-stub}} | {{Chem-stub}} | ||
<gallery> | |||
File:Phthalic-acid-2D-skeletal.png|2D Skeletal Structure of Phthalic Acid | |||
File:Phthalic_acid_3D_ball.png|3D Ball Model of Phthalic Acid | |||
File:Phthalic_acid.JPG|Phthalic Acid | |||
</gallery> | |||
Latest revision as of 01:31, 18 February 2025
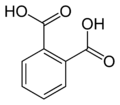
Phthalic acid is an aromatic dicarboxylic acid, with formula C6H4(COOH)2. It is an isomer of isophthalic acid and terephthalic acid. Phthalic acid is an industrially important chemical used primarily in the production of plasticizers for plastics.
History[edit]
Phthalic acid was first obtained by French chemist Auguste Laurent in 1836 by oxidizing naphthalene in the presence of aluminium chloride. However, it was not until 1863 that German chemist August Wilhelm von Hofmann correctly identified the formula of phthalic acid.
Production[edit]
Phthalic acid is produced by the catalytic oxidation of naphthalene or ortho-xylene directly by oxygen in the presence of a vanadium pentoxide catalyst.
Uses[edit]
The primary use of phthalic acid is in the production of plasticizers, specifically phthalates, that increase the flexibility, transparency, durability, and longevity of plastics. It is also used as a precursor to other chemicals such as phthalimide and phthalanilic acid.
Health and Safety[edit]
Phthalic acid is a relatively weak acid and is generally safe to handle. However, it can cause irritation to the skin and eyes. Long-term exposure to phthalic acid can lead to more serious health problems such as respiratory disorders and endocrine disruption.
Environmental Impact[edit]
Phthalic acid and its derivatives have been detected in the environment due to their widespread use in plastics and other industrial processes. They are known to be endocrine disruptors, and their potential effects on human health and the environment are the subject of ongoing research.
See Also[edit]
-
2D Skeletal Structure of Phthalic Acid
-
3D Ball Model of Phthalic Acid
-
Phthalic Acid