Phenyl acetate: Difference between revisions
CSV import |
CSV import |
||
| Line 24: | Line 24: | ||
[[Category:Chemical compounds]] | [[Category:Chemical compounds]] | ||
{{Chem-stub}} | {{Chem-stub}} | ||
==Phenyl_acetate== | |||
<gallery> | |||
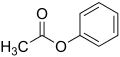
File:Phenyl_acetate_200.svg|Phenyl acetate structural formula | |||
File:Phenyl_acetate_3D_ball.png|Phenyl acetate 3D ball model | |||
</gallery> | |||
Latest revision as of 00:54, 18 February 2025
Phenyl acetate is an organic compound with the chemical formula C8H8O2. It is the ester formed from acetic acid and phenol. Phenyl acetate is a colorless liquid that is slightly soluble in water but more soluble in organic solvents such as ethanol, diethyl ether, and benzene. It has a distinctive sweet and honey-like odor, making it a valuable ingredient in the flavor and fragrance industry.
Properties[edit]
Phenyl acetate has a molecular weight of 136.15 g/mol. It boils at 195.5°C and has a density of 1.0804 g/cm3. Its refractive index is 1.5060. The compound is relatively stable but can hydrolyze in the presence of strong bases or acids to form acetic acid and phenol.
Synthesis[edit]
Phenyl acetate can be synthesized through the esterification of acetic acid with phenol. This reaction is typically catalyzed by an acid such as sulfuric acid:
C6H5OH + CH3COOH → C6H5OOCCH3 + H2O
Another method involves the reaction of phenol with acetyl chloride or acetic anhydride.
Applications[edit]
Phenyl acetate is used in the synthesis of pharmaceuticals, including some penicillin derivatives. It is also employed as a solvent and an intermediate in the manufacture of dyes, perfumes, and flavorings. Due to its pleasant aroma, it is used in the formulation of fragrances and as a flavoring agent in food products.
Safety[edit]
Phenyl acetate is considered to be moderately toxic. It can cause irritation to the skin, eyes, and respiratory tract. Ingestion or prolonged exposure can lead to more severe health effects. Proper handling and protective equipment are recommended when working with this chemical.
Environmental Impact[edit]
While phenyl acetate is not classified as a major environmental pollutant, it should be disposed of properly to minimize its impact on the environment. It is biodegradable under aerobic conditions, but its breakdown products can still pose risks to aquatic and terrestrial life.
Phenyl_acetate[edit]
-
Phenyl acetate structural formula
-
Phenyl acetate 3D ball model