Ketene: Difference between revisions
CSV import |
CSV import |
||
| Line 22: | Line 22: | ||
{{chemistry-stub}} | {{chemistry-stub}} | ||
<gallery> | |||
File:Ketenes_nonsymmetric.png|Ketene | |||
File:Mecanisme-de-la-formation-des-cetenes.png|Ketene | |||
File:Ketene_Synthesis.png|Ketene | |||
File:Ketene_Reaktion1_V1.svg|Ketene | |||
File:Ketene_Reaktion2_V1.svg|Ketene | |||
File:Ketene_Reaktion4_V1.svg|Ketene | |||
File:Ketene_Reaktion6_V1.svg|Ketene | |||
File:Ketene_Reaktion7_V3_unlabeled.svg|Ketene | |||
File:Dimerisation_of_ketene.png|Ketene | |||
File:Staudinger-Synthese_ÜV6.svg|Ketene | |||
</gallery> | |||
Latest revision as of 11:46, 18 February 2025

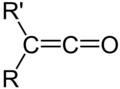
Ketene is an organic compound with the chemical formula C2H2O. It is a highly reactive ethylene derivative and is the simplest member of the ketenes, a group of compounds characterized by the functional group R2C=C=O, where R can be a variety of carbon-containing substituents. Ketenes are used in the production of acetic acid, acetic anhydride, and various acetyl derivatives, serving as important intermediates in organic synthesis and industrial chemistry.
Properties and Structure[edit]
Ketene is a colorless, poisonous gas with a pungent odor. It is extremely reactive due to the presence of a strained double bond between the carbon and oxygen atoms, making it an efficient acylating agent. The molecule consists of a planar arrangement of atoms, with the central carbon atom double bonded to both an oxygen atom and another carbon atom, forming a linear structure. This configuration contributes to its high reactivity, particularly in addition reactions with nucleophiles.
Synthesis[edit]
Ketene is typically synthesized through the pyrolysis of acetic acid or acetone. The process involves heating these substances to a high temperature, causing a decomposition reaction that produces ketene along with other byproducts. Another method involves the dehalogenation of dichloroethene in the presence of a strong base.
Reactions[edit]
Due to its high reactivity, ketene readily undergoes addition reactions with a variety of nucleophiles, including water, alcohols, and amines, to form carboxylic acids, esters, and amides, respectively. These reactions are often exploited in organic synthesis to introduce acyl groups into molecules.
Applications[edit]
Ketene's reactivity makes it a valuable intermediate in the chemical industry. It is used in the synthesis of acetic acid, one of the most important industrial chemicals, through its hydration. Ketene also reacts with alcohols to produce esters, which are important in the manufacture of plastics, solvents, and pharmaceuticals. Additionally, its reaction with amines yields amides, which are used in the production of plastics and rubber.
Safety[edit]
Ketene is highly toxic and can cause severe respiratory issues upon inhalation. It is also flammable, posing a risk of fire and explosion. Proper safety measures, including adequate ventilation and the use of protective equipment, are essential when handling ketene.
-
Ketene
-
Ketene
-
Ketene
-
Ketene
-
Ketene
-
Ketene
-
Ketene
-
Ketene
-
Ketene
-
Ketene