Chemical structure: Difference between revisions
CSV import |
CSV import |
||
| Line 32: | Line 32: | ||
[[Category:Chemical bonding]] | [[Category:Chemical bonding]] | ||
{{Chemistry-stub}} | {{Chemistry-stub}} | ||
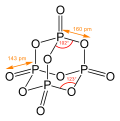
<gallery> | |||
File:Phosphorus-pentoxide-2D-dimensions.svg | |||
</gallery> | |||
Latest revision as of 21:59, 16 February 2025
Chemical structure refers to the spatial arrangement of atoms and the chemical bonds that hold the atoms together. The chemical structure of a molecule can significantly influence its physical and chemical properties, including its reactivity, polarity, phase of matter, color, magnetism, and biological activity.
Overview[edit]
The chemical structure of a molecule can be described in terms of its atoms, the ways those atoms are arranged, and the chemical bonds that hold the atoms together. The chemical structure can be represented graphically as a structural formula.
Atoms[edit]
Atoms are the basic units of matter and the defining structure of elements. They are composed of three kinds of subatomic particles: protons, neutrons, and electrons. The protons and neutrons form the nucleus of the atom, while the electrons orbit the nucleus.
Bonds[edit]
Chemical bonds are the attractive forces that hold atoms together in molecules. There are several types of chemical bonds, including covalent bonds, ionic bonds, and hydrogen bonds.
Structural Formula[edit]
A structural formula is a type of chemical formula that shows the arrangement of atoms in a molecule and the bonds between them. The structural formula can be represented in several ways, including the Lewis structure, the line-angle formula, and the ball-and-stick model.
Chemical Properties[edit]
The chemical structure of a molecule determines its chemical properties, including its reactivity, polarity, phase of matter, color, magnetism, and biological activity. For example, the chemical structure of water (H2O) makes it a polar molecule, which means it has a positive charge on one side and a negative charge on the other.
See Also[edit]