2-Deoxy-D-glucose: Difference between revisions
CSV import |
CSV import Tags: mobile edit mobile web edit |
||
| Line 1: | Line 1: | ||
{{Short description| | {{Short description|Overview of 2-Deoxy-D-glucose}} | ||
{{ | {{DISPLAYTITLE:2-Deoxy-D-glucose}} | ||
== 2-Deoxy-D-glucose == | |||
[[File:2-Deoxy-D-glucose.png|thumb|right|Chemical structure of 2-Deoxy-D-glucose]] | |||
2-Deoxy-D-glucose (2-DG) is a [[glucose]] [[molecule]] that has been modified by the removal of an oxygen atom from the second carbon. This alteration prevents it from undergoing further glycolysis, making it a useful tool in [[biochemical]] research and a potential therapeutic agent. | |||
== | == Chemical Properties == | ||
2-Deoxy-D-glucose is | 2-Deoxy-D-glucose is a [[glucose analog]] that differs from normal glucose by the absence of a hydroxyl group at the C-2 position. This structural change is crucial as it inhibits the molecule's ability to proceed through the [[glycolytic pathway]]. | ||
==Mechanism of Action== | == Mechanism of Action == | ||
2- | 2-DG is taken up by cells via the same transporters as glucose. Once inside the cell, it is phosphorylated by [[hexokinase]] to form 2-deoxy-D-glucose-6-phosphate. However, this compound cannot be further metabolized by [[phosphoglucose isomerase]], leading to an accumulation of 2-DG-6-phosphate and a subsequent decrease in glycolysis and ATP production. | ||
== | == Applications in Research == | ||
2-Deoxy-D-glucose is widely used in [[metabolic studies]] to investigate the role of glycolysis in various cellular processes. It is also employed in [[cancer research]] to study the [[Warburg effect]], where cancer cells preferentially utilize glycolysis for energy production even in the presence of oxygen. | |||
== | == Therapeutic Potential == | ||
2- | The ability of 2-DG to inhibit glycolysis has led to its investigation as a potential [[anticancer agent]]. By targeting the altered metabolic pathways in cancer cells, 2-DG may selectively inhibit tumor growth while sparing normal cells. | ||
== | == Safety and Side Effects == | ||
While 2-DG shows promise in preclinical studies, its use in humans is associated with potential side effects such as hypoglycemia and gastrointestinal discomfort. Further research is needed to fully understand its safety profile and therapeutic window. | |||
== | == Related Pages == | ||
* [[Glycolysis]] | |||
* [[Glucose metabolism]] | * [[Glucose metabolism]] | ||
* [[Cancer | * [[Cancer metabolism]] | ||
* [[ | * [[Hexokinase]] | ||
[[Category:Glucose]] | [[Category:Glucose analogs]] | ||
[[Category: | [[Category:Biochemistry]] | ||
[[Category: | [[Category:Experimental cancer treatments]] | ||
Latest revision as of 11:13, 15 February 2025
Overview of 2-Deoxy-D-glucose
2-Deoxy-D-glucose[edit]
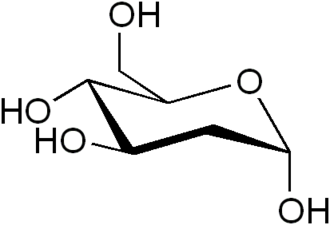
2-Deoxy-D-glucose (2-DG) is a glucose molecule that has been modified by the removal of an oxygen atom from the second carbon. This alteration prevents it from undergoing further glycolysis, making it a useful tool in biochemical research and a potential therapeutic agent.
Chemical Properties[edit]
2-Deoxy-D-glucose is a glucose analog that differs from normal glucose by the absence of a hydroxyl group at the C-2 position. This structural change is crucial as it inhibits the molecule's ability to proceed through the glycolytic pathway.
Mechanism of Action[edit]
2-DG is taken up by cells via the same transporters as glucose. Once inside the cell, it is phosphorylated by hexokinase to form 2-deoxy-D-glucose-6-phosphate. However, this compound cannot be further metabolized by phosphoglucose isomerase, leading to an accumulation of 2-DG-6-phosphate and a subsequent decrease in glycolysis and ATP production.
Applications in Research[edit]
2-Deoxy-D-glucose is widely used in metabolic studies to investigate the role of glycolysis in various cellular processes. It is also employed in cancer research to study the Warburg effect, where cancer cells preferentially utilize glycolysis for energy production even in the presence of oxygen.
Therapeutic Potential[edit]
The ability of 2-DG to inhibit glycolysis has led to its investigation as a potential anticancer agent. By targeting the altered metabolic pathways in cancer cells, 2-DG may selectively inhibit tumor growth while sparing normal cells.
Safety and Side Effects[edit]
While 2-DG shows promise in preclinical studies, its use in humans is associated with potential side effects such as hypoglycemia and gastrointestinal discomfort. Further research is needed to fully understand its safety profile and therapeutic window.