4'-Fluorocannabidiol: Difference between revisions
CSV import |
CSV import |
||
| (2 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{DISPLAYTITLE:4'-Fluorocannabidiol (HUF-101)}} | |||
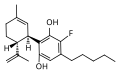
{{DISPLAYTITLE:4'-Fluorocannabidiol}} | 4'-Fluorocannabidiol, also known as HUF-101, is a synthetic derivative of [[cannabidiol]] (CBD), a major non-psychoactive component of the [[Cannabis sativa]] plant. HUF-101 is characterized by the substitution of a fluorine atom at the 4' position of the cannabidiol molecule, which may alter its pharmacological properties. | ||
== Chemical Properties == | |||
HUF-101 is a fluorinated analog of cannabidiol, which means it has a similar chemical structure with the addition of a fluorine atom. This modification can influence the compound's [[lipophilicity]], [[metabolic stability]], and [[binding affinity]] to various [[receptors]] in the body. | |||
== | == Pharmacology == | ||
4'-Fluorocannabidiol is | The pharmacological profile of 4'-Fluorocannabidiol is of interest due to its potential therapeutic effects. Like cannabidiol, HUF-101 is thought to interact with the [[endocannabinoid system]], although its precise mechanism of action and receptor targets may differ due to the presence of the fluorine atom. | ||
== | === Potential Therapeutic Uses === | ||
Research into HUF-101 is ongoing, with studies exploring its potential use in treating conditions such as [[epilepsy]], [[anxiety disorders]], and [[inflammation]]. The fluorination of the molecule may enhance its efficacy or alter its side effect profile compared to non-fluorinated cannabidiol. | |||
== | == Synthesis == | ||
The synthesis of 4'-Fluorocannabidiol involves the introduction of a fluorine atom into the cannabidiol structure. This process requires specialized chemical techniques to ensure the correct positioning of the fluorine atom and to maintain the integrity of the cannabidiol framework. | |||
== | == Safety and Legal Status == | ||
As a synthetic cannabinoid, the safety profile of HUF-101 is not as well-established as that of naturally occurring cannabidiol. Its legal status may vary by jurisdiction, depending on local regulations regarding synthetic cannabinoids and fluorinated compounds. | |||
== Related Pages == | |||
==Related | |||
* [[Cannabidiol]] | * [[Cannabidiol]] | ||
* [[Cannabis sativa]] | |||
* [[Endocannabinoid system]] | |||
* [[Synthetic cannabinoids]] | * [[Synthetic cannabinoids]] | ||
[[Category:Cannabinoids]] | |||
[[Category:Synthetic drugs]] | |||
[[Category:Fluorinated compounds]] | |||
<gallery> | <gallery> | ||
File:HUF-101.svg| | File:HUF-101.svg|4'-Fluorocannabidiol | ||
</gallery> | </gallery> | ||
Latest revision as of 01:11, 20 February 2025
4'-Fluorocannabidiol, also known as HUF-101, is a synthetic derivative of cannabidiol (CBD), a major non-psychoactive component of the Cannabis sativa plant. HUF-101 is characterized by the substitution of a fluorine atom at the 4' position of the cannabidiol molecule, which may alter its pharmacological properties.
Chemical Properties[edit]
HUF-101 is a fluorinated analog of cannabidiol, which means it has a similar chemical structure with the addition of a fluorine atom. This modification can influence the compound's lipophilicity, metabolic stability, and binding affinity to various receptors in the body.
Pharmacology[edit]
The pharmacological profile of 4'-Fluorocannabidiol is of interest due to its potential therapeutic effects. Like cannabidiol, HUF-101 is thought to interact with the endocannabinoid system, although its precise mechanism of action and receptor targets may differ due to the presence of the fluorine atom.
Potential Therapeutic Uses[edit]
Research into HUF-101 is ongoing, with studies exploring its potential use in treating conditions such as epilepsy, anxiety disorders, and inflammation. The fluorination of the molecule may enhance its efficacy or alter its side effect profile compared to non-fluorinated cannabidiol.
Synthesis[edit]
The synthesis of 4'-Fluorocannabidiol involves the introduction of a fluorine atom into the cannabidiol structure. This process requires specialized chemical techniques to ensure the correct positioning of the fluorine atom and to maintain the integrity of the cannabidiol framework.
Safety and Legal Status[edit]
As a synthetic cannabinoid, the safety profile of HUF-101 is not as well-established as that of naturally occurring cannabidiol. Its legal status may vary by jurisdiction, depending on local regulations regarding synthetic cannabinoids and fluorinated compounds.
Related Pages[edit]
-
4'-Fluorocannabidiol