Clavulanic acid: Difference between revisions
CSV import |
CSV import |
||
| Line 23: | Line 23: | ||
{{Medicine-stub}} | {{Medicine-stub}} | ||
== Clavulanic_acid == | |||
<gallery> | |||
File:Clavulanic_acid.svg|Chemical structure of Clavulanic acid | |||
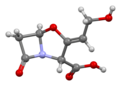
File:Clavulanic-acid-based-on-xtal-3D-bs-17.png|3D model of Clavulanic acid | |||
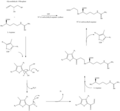
File:Clavualnic_Acid_Biosynthesis.png|Biosynthesis pathway of Clavulanic acid | |||
File:Beta-lactam_synthetase_mechanism.png|Mechanism of Beta-lactam synthetase | |||
File:CEA_synthase_mechanism.png|Mechanism of CEA synthase | |||
</gallery> | |||
Latest revision as of 05:01, 18 February 2025
Clavulanic Acid is a beta-lactamase inhibitor that enhances the effectiveness of beta-lactam antibiotics against bacteria that would otherwise be resistant. It is structurally related to the penicillins and possesses the ability to inactivate a wide variety of beta-lactamase enzymes found in bacteria resistant to penicillins and cephalosporins. Clavulanic acid, by itself, does not possess any significant antibacterial activity. However, when combined with certain penicillin-type antibiotics, it can expand their spectrum of activity to include bacteria normally resistant to them.
Chemistry[edit]
Clavulanic acid is produced by the fermentation of the bacterium Streptomyces clavuligerus. It is a beta-lactam compound that shares a similar structure to penicillin. The molecular formula of clavulanic acid is C8H9NO5, and it functions primarily by binding to and permanently deactivating beta-lactamase enzymes, thus protecting beta-lactam antibiotics from degradation.
Mechanism of Action[edit]
The primary mechanism of action of clavulanic acid is the inhibition of beta-lactamase enzymes. These enzymes are produced by bacteria as a defense mechanism against beta-lactam antibiotics. By binding to the active sites of these enzymes, clavulanic acid prevents them from breaking down the antibiotic molecule, allowing the antibiotic to remain active against the bacteria.
Clinical Uses[edit]
Clavulanic acid is used in combination with amoxicillin (as Co-amoxiclav) or ticarcillin. These combinations are effective against a broad range of bacteria, including Staphylococcus aureus, Escherichia coli, and Klebsiella pneumoniae, which might otherwise be resistant. The combinations are used to treat various infections, including respiratory tract infections, urinary tract infections, skin infections, and more.
Side Effects[edit]
The use of clavulanic acid can be associated with several side effects, most commonly gastrointestinal disturbances such as diarrhea, nausea, and vomiting. As with all medications, allergic reactions can occur but are less common. Liver dysfunction has been reported in a small number of cases, usually reversible upon discontinuation of the drug.
Resistance[edit]
While clavulanic acid is a powerful inhibitor of beta-lactamase enzymes, bacterial resistance can still develop. Some bacteria produce beta-lactamase enzymes that clavulanic acid cannot inhibit effectively, leading to treatment failure. Continuous monitoring and research are necessary to understand resistance mechanisms and develop new inhibitors.
Conclusion[edit]
Clavulanic acid represents a significant advancement in the fight against bacterial resistance to beta-lactam antibiotics. Its discovery and use in combination therapies have broadened the spectrum of bacterial infections that can be effectively treated. Ongoing research into beta-lactamase inhibitors and resistance mechanisms is essential to stay ahead in the battle against bacterial infections.
Clavulanic_acid[edit]
-
Chemical structure of Clavulanic acid
-
3D model of Clavulanic acid
-
Biosynthesis pathway of Clavulanic acid
-
Mechanism of Beta-lactam synthetase
-
Mechanism of CEA synthase