Γ-Butyrolactone: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| Line 24: | Line 24: | ||
[[Category:Solvents]] | [[Category:Solvents]] | ||
{{Chemistry-stub}} | {{Chemistry-stub}} | ||
== Γ-Butyrolactone == | |||
<gallery> | |||
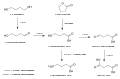
File:Industrial_synthesis_of_gamma-butyrolactone.svg|Industrial synthesis of gamma-butyrolactone | |||
File:MCPB.png|MCPB | |||
File:GHB_metabolic_pathway.svg|GHB metabolic pathway | |||
File:GBLjugs.jpg|GBL jugs | |||
File:Dangerous_dietary_supplements.gif|Dangerous dietary supplements | |||
</gallery> | |||
Latest revision as of 23:58, 24 February 2025
Γ-Butyrolactone (GBL) is a hygroscopic colorless liquid with a weak characteristic odor and profoundly bitter taste. It is a common solvent and reagent in chemistry and is used as an aroma compound, as a stain remover, as a superglue remover, and as a solvent in some wet aluminium electrolyte capacitors.
Chemical Properties[edit]
GBL is a lactone. It is hydrolyzed under basic conditions, for example in a sodium hydroxide solution into sodium gamma-hydroxybutyrate, the sodium salt of gamma-hydroxybutyric acid. Under acidic conditions, it forms an equilibrium mixture of both compounds. These compounds then may go on to form the polymer poly(4-hydroxybutyrate).
Uses[edit]
GBL is not active in its own right; its mechanism of action stems from its identity as a prodrug of gamma-hydroxybutyric acid (GHB). The hypnotic effect of GHB is enhanced by combination with alcohol. A 2003 rat study showed that GBL in combination with ethanol showed a potentiated hypnotic effect, as the sleep-timing measure was longer than both of the individual components combined.
Safety[edit]
Overdose of GBL can cause adverse effects, including sedation, vertigo, nausea, and death. GBL has a distinctive taste and odor, described as being comparable to stale water, synthetic melon aroma, or burnt plastic. This differs significantly from GHB, which is described as having a decidedly salty taste. People who ingest GBL may not be aware they are consuming a drug.
Legal Status[edit]
In many countries, including the United States and Canada, the production, sale, and possession of GBL are illegal since it can be used as a prodrug of GHB. In the United Kingdom, GBL is classified as a Class C drug.
See Also[edit]
Γ-Butyrolactone[edit]
-
Industrial synthesis of gamma-butyrolactone
-
MCPB
-
GHB metabolic pathway
-
GBL jugs
-
Dangerous dietary supplements