Methestrol: Difference between revisions
CSV import |
CSV import |
||
| Line 30: | Line 30: | ||
[[Category:Abandoned drugs]] | [[Category:Abandoned drugs]] | ||
{{Pharma-stub}} | {{Pharma-stub}} | ||
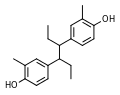
<gallery> | |||
File:Methestrol.svg|Methestrol | |||
</gallery> | |||
Latest revision as of 01:27, 20 February 2025
Methestrol (also known as promethestrol) is a synthetic, nonsteroidal estrogen that was formerly used for estrogen replacement therapy and is now no longer marketed. It is an estrogen ethanolamine ester that was first described in the literature in 1938 and was introduced for medical use by 1941.
History[edit]
Methestrol was first synthesized in 1938 by Wilhelm Collani and his team at Schering AG in Berlin. It was one of the first synthetic estrogens to be developed, and it was introduced for medical use by 1941. Methestrol was used for a variety of indications, including menopausal symptoms, gynecological disorders, and cancers.
Pharmacology[edit]
As an estrogen, methestrol has similar effects to natural estrogens in the body. It binds to and activates the estrogen receptor, which leads to changes in gene expression and produces estrogenic effects. Methestrol is a nonsteroidal estrogen, which means it does not have a steroid structure like natural estrogens do. Instead, it has a synthetic structure that was designed to mimic the effects of natural estrogens.
Side Effects[edit]
Like other estrogens, methestrol can have a number of side effects. These can include nausea, vomiting, breast tenderness, menstrual irregularities, and fluid retention. In addition, long-term use of methestrol can increase the risk of certain types of cancer, including breast cancer and endometrial cancer.
Current Status[edit]
Methestrol is no longer marketed and is not currently used in medicine. It was withdrawn from the market due to its side effects and the availability of safer and more effective estrogen replacement therapies.
See Also[edit]
-
Methestrol