Saquinavir: Difference between revisions
CSV import |
CSV import |
||
| (One intermediate revision by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{Short description|Antiviral medication used to treat HIV/AIDS}} | ||
{{Drugbox | |||
| verifiedfields = changed | |||
| verifiedrevid = 477241870 | |||
| IUPAC_name = (2S)-N-((2S,3R)-4-((2S)-2-((2S,3R)-3-hydroxy-4-phenylbutan-2-ylamino)-3-methylbutanoyl)amino)-3-hydroxy-1-phenylbutan-2-yl)-2-(quinolin-2-ylformamido)butanediamide | |||
| image = Saquinavir_structure.svg | |||
| image2 = Saquinavir_ball-and-stick.png | |||
}} | |||
'''Saquinavir''' is an [[antiretroviral drug]] used in the treatment of [[HIV/AIDS]]. It belongs to a class of medications known as [[protease inhibitors]], which work by inhibiting the action of the [[HIV-1 protease]], an enzyme critical for the maturation of infectious virus particles. Saquinavir was the first protease inhibitor approved by the [[Food and Drug Administration]] (FDA) in December 1995. | |||
Saquinavir | |||
==Mechanism of Action== | |||
Saquinavir | Saquinavir functions by binding to the active site of the HIV-1 protease enzyme, preventing the cleavage of the [[gag-pol polyprotein]], which is necessary for the production of mature viral particles. This inhibition results in the production of immature, non-infectious viral particles, thereby reducing the viral load in the patient's body. | ||
==Pharmacokinetics== | |||
Saquinavir is typically administered in combination with other antiretroviral agents to enhance its efficacy. It is poorly absorbed when taken alone, but its bioavailability is significantly increased when co-administered with [[ritonavir]], another protease inhibitor that acts as a pharmacokinetic enhancer by inhibiting the [[cytochrome P450 3A4]] enzyme. | |||
==Clinical Use== | |||
Saquinavir is used as part of [[highly active antiretroviral therapy]] (HAART) for the treatment of HIV infection. It is often prescribed in combination with other antiretroviral drugs to achieve optimal viral suppression and to prevent the development of drug resistance. | |||
==Side Effects== | |||
Common side effects of saquinavir include gastrointestinal disturbances such as nausea, diarrhea, and abdominal pain. It may also cause [[lipodystrophy]], a condition characterized by changes in body fat distribution. Patients on saquinavir therapy should be monitored for potential liver toxicity and [[hyperglycemia]]. | |||
==Resistance== | |||
== | Resistance to saquinavir can develop through mutations in the HIV-1 protease gene, which may reduce the drug's binding affinity to the enzyme. Cross-resistance with other protease inhibitors can also occur, necessitating careful selection of antiretroviral regimens in patients with resistant virus strains. | ||
==History== | |||
Saquinavir was developed by [[Hoffmann-La Roche]] and was the first protease inhibitor to be approved by the FDA. Its approval marked a significant advancement in the treatment of HIV/AIDS, leading to the development of more effective combination therapies that have transformed HIV from a fatal disease to a manageable chronic condition. | |||
File: | |||
==Impact on HIV/AIDS Epidemic== | |||
[[File:HIV_new_infections_and_deaths_1981-2008.jpg|thumb|right|HIV new infections and deaths 1981-2008]] | |||
The introduction of saquinavir and other protease inhibitors in the mid-1990s significantly reduced the mortality and morbidity associated with HIV/AIDS. The widespread use of HAART has led to a dramatic decline in AIDS-related deaths and has improved the quality of life for many individuals living with HIV. | |||
==Related Pages== | |||
* [[HIV/AIDS]] | |||
* [[Protease inhibitor (pharmacology)]] | |||
* [[Antiretroviral drug]] | |||
* [[Highly active antiretroviral therapy]] | |||
[[Category:Antiviral drugs]] | |||
[[Category:Protease inhibitors]] | |||
[[Category:Hoffmann-La Roche brands]] | |||
Latest revision as of 11:16, 23 March 2025
Antiviral medication used to treat HIV/AIDS
| Saquinavir | |
|---|---|
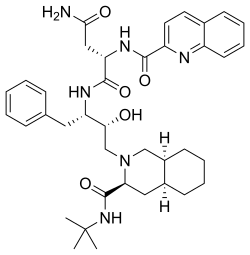
| |
| INN | |
| Drug class | |
| Routes of administration | |
| Pregnancy category | |
| Bioavailability | |
| Metabolism | |
| Elimination half-life | |
| Excretion | |
| Legal status | |
| CAS Number | |
| PubChem | |
| DrugBank | |
| ChemSpider | |
| KEGG | |
Saquinavir is an antiretroviral drug used in the treatment of HIV/AIDS. It belongs to a class of medications known as protease inhibitors, which work by inhibiting the action of the HIV-1 protease, an enzyme critical for the maturation of infectious virus particles. Saquinavir was the first protease inhibitor approved by the Food and Drug Administration (FDA) in December 1995.
Mechanism of Action[edit]
Saquinavir functions by binding to the active site of the HIV-1 protease enzyme, preventing the cleavage of the gag-pol polyprotein, which is necessary for the production of mature viral particles. This inhibition results in the production of immature, non-infectious viral particles, thereby reducing the viral load in the patient's body.
Pharmacokinetics[edit]
Saquinavir is typically administered in combination with other antiretroviral agents to enhance its efficacy. It is poorly absorbed when taken alone, but its bioavailability is significantly increased when co-administered with ritonavir, another protease inhibitor that acts as a pharmacokinetic enhancer by inhibiting the cytochrome P450 3A4 enzyme.
Clinical Use[edit]
Saquinavir is used as part of highly active antiretroviral therapy (HAART) for the treatment of HIV infection. It is often prescribed in combination with other antiretroviral drugs to achieve optimal viral suppression and to prevent the development of drug resistance.
Side Effects[edit]
Common side effects of saquinavir include gastrointestinal disturbances such as nausea, diarrhea, and abdominal pain. It may also cause lipodystrophy, a condition characterized by changes in body fat distribution. Patients on saquinavir therapy should be monitored for potential liver toxicity and hyperglycemia.
Resistance[edit]
Resistance to saquinavir can develop through mutations in the HIV-1 protease gene, which may reduce the drug's binding affinity to the enzyme. Cross-resistance with other protease inhibitors can also occur, necessitating careful selection of antiretroviral regimens in patients with resistant virus strains.
History[edit]
Saquinavir was developed by Hoffmann-La Roche and was the first protease inhibitor to be approved by the FDA. Its approval marked a significant advancement in the treatment of HIV/AIDS, leading to the development of more effective combination therapies that have transformed HIV from a fatal disease to a manageable chronic condition.
Impact on HIV/AIDS Epidemic[edit]

The introduction of saquinavir and other protease inhibitors in the mid-1990s significantly reduced the mortality and morbidity associated with HIV/AIDS. The widespread use of HAART has led to a dramatic decline in AIDS-related deaths and has improved the quality of life for many individuals living with HIV.