Nylon: Difference between revisions
CSV import |
CSV import |
||
| Line 1: | Line 1: | ||
==Nylon== | |||
[[File:Nylon_6_and_Nylon_6-6.svg|Nylon 6 and Nylon 6-6 structure|thumb|right]] | |||
Nylon | '''Nylon''' is a generic designation for a family of synthetic polymers known as [[polyamides]]. It was first produced on February 28, 1935, by Wallace Carothers at [[DuPont]], a major American chemical company. Nylon is a thermoplastic silky material that can be melt-processed into fibers, films, or shapes. It is one of the most widely used polymers. | ||
== | ==History== | ||
[[File:Wallace_Carothers,_in_the_lab.jpg|Wallace Carothers in the lab|thumb|left]] | |||
The development of nylon began in the early 1930s when Wallace Carothers, a chemist at DuPont, was tasked with creating a synthetic fiber that could replace silk. Carothers and his team successfully synthesized the first nylon polymer in 1935. The first commercial use of nylon was in a nylon-bristled toothbrush in 1938, followed by women's stockings in 1940, which became immensely popular. | |||
== | ==Chemical Structure== | ||
Nylon is | Nylon is a polyamide, which means it contains repeating units linked by amide bonds. The most common types of nylon are [[Nylon 6]] and [[Nylon 6,6]]. Nylon 6 is made from a single type of monomer, caprolactam, while Nylon 6,6 is made from two monomers, hexamethylenediamine and adipic acid. The polymerization process involves a condensation reaction, where water is released as a byproduct. | ||
[[File:Condensation_polymerization_diacid_diamine.svg|Condensation polymerization of diacid and diamine|thumb|right]] | |||
== | ==Properties== | ||
Nylon | Nylon is known for its high tensile strength, elasticity, and resistance to abrasion and chemicals. It is also resistant to heat and can be dyed easily. These properties make it suitable for a wide range of applications, from clothing to industrial uses. | ||
== | ==Applications== | ||
[[File:NMA.0028271,_Fashion_Photo_by_Erik_Liljeroth_1954.jpg|Fashion Photo by Erik Liljeroth, 1954|thumb|left]] | |||
Nylon is used in a variety of applications, including: | |||
* '''Textiles and Fabrics''': Nylon is used in the production of hosiery, swimwear, activewear, and other clothing items due to its elasticity and strength. | |||
* '''Industrial Uses''': It is used in the manufacture of ropes, conveyor belts, and automotive parts. | |||
* '''Consumer Goods''': Nylon is used in toothbrush bristles, fishing lines, and guitar strings. | |||
==Environmental Impact== | |||
The production of nylon is energy-intensive and involves the use of petrochemicals, which contribute to environmental pollution. However, efforts are being made to recycle nylon products and develop more sustainable production methods. | |||
[[File:The_worn_out_nylon_stockings_in_this_barrel_full_of_salvaged_stockings_will_be_reprocessed_and_made_into_parachutes..._-_NARA_-_196427.jpg|Worn out nylon stockings for reprocessing|thumb|right]] | |||
==Microstructure== | |||
[[File:Nylon_fibre_SEM.tif|Nylon fibre SEM|thumb|left]] | |||
Nylon fibers have a crystalline structure that contributes to their strength and durability. The hydrogen bonds between the polymer chains enhance the material's mechanical properties. | |||
[[File:Nylon-3D-h_bond.png|Nylon 3D hydrogen bond|thumb|right]] | |||
==Related Pages== | |||
* [[Polymer]] | * [[Polymer]] | ||
* [[ | * [[Polyamide]] | ||
* [[Wallace Carothers]] | |||
* [[Wallace | |||
* [[DuPont]] | * [[DuPont]] | ||
[[Category:Synthetic fibers]] | [[Category:Synthetic fibers]] | ||
[[Category: | [[Category:Polyamides]] | ||
[[Category:DuPont]] | [[Category:DuPont]] | ||
Latest revision as of 10:48, 23 March 2025
Nylon[edit]

Nylon is a generic designation for a family of synthetic polymers known as polyamides. It was first produced on February 28, 1935, by Wallace Carothers at DuPont, a major American chemical company. Nylon is a thermoplastic silky material that can be melt-processed into fibers, films, or shapes. It is one of the most widely used polymers.
History[edit]

The development of nylon began in the early 1930s when Wallace Carothers, a chemist at DuPont, was tasked with creating a synthetic fiber that could replace silk. Carothers and his team successfully synthesized the first nylon polymer in 1935. The first commercial use of nylon was in a nylon-bristled toothbrush in 1938, followed by women's stockings in 1940, which became immensely popular.
Chemical Structure[edit]
Nylon is a polyamide, which means it contains repeating units linked by amide bonds. The most common types of nylon are Nylon 6 and Nylon 6,6. Nylon 6 is made from a single type of monomer, caprolactam, while Nylon 6,6 is made from two monomers, hexamethylenediamine and adipic acid. The polymerization process involves a condensation reaction, where water is released as a byproduct.

Properties[edit]
Nylon is known for its high tensile strength, elasticity, and resistance to abrasion and chemicals. It is also resistant to heat and can be dyed easily. These properties make it suitable for a wide range of applications, from clothing to industrial uses.
Applications[edit]

Nylon is used in a variety of applications, including:
- Textiles and Fabrics: Nylon is used in the production of hosiery, swimwear, activewear, and other clothing items due to its elasticity and strength.
- Industrial Uses: It is used in the manufacture of ropes, conveyor belts, and automotive parts.
- Consumer Goods: Nylon is used in toothbrush bristles, fishing lines, and guitar strings.
Environmental Impact[edit]
The production of nylon is energy-intensive and involves the use of petrochemicals, which contribute to environmental pollution. However, efforts are being made to recycle nylon products and develop more sustainable production methods.

Microstructure[edit]
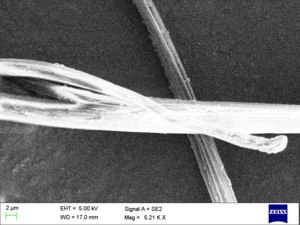
Nylon fibers have a crystalline structure that contributes to their strength and durability. The hydrogen bonds between the polymer chains enhance the material's mechanical properties.
