Xylene: Difference between revisions
CSV import |
No edit summary |
||
| (5 intermediate revisions by the same user not shown) | |||
| Line 13: | Line 13: | ||
Xylene poses a risk of fire and explosion. It is harmful if inhaled, and irritating to the eyes, skin, and respiratory tract. It may cause effects on the central nervous system, resulting in impaired functions. | Xylene poses a risk of fire and explosion. It is harmful if inhaled, and irritating to the eyes, skin, and respiratory tract. It may cause effects on the central nervous system, resulting in impaired functions. | ||
== Xylene gallery == | |||
<gallery> | |||
File:IUPAC-cyclic.svg|IUPAC cyclic | |||
</gallery> | |||
==See Also== | ==See Also== | ||
* [[Toluene]] | * [[Toluene]] | ||
* [[Benzene]] | * [[Benzene]] | ||
* [[Solvent]] | * [[Solvent]] | ||
[[Category:Chemical compounds]] | [[Category:Chemical compounds]] | ||
[[Category:Solvents]] | [[Category:Solvents]] | ||
[[Category:Industrial chemicals]] | [[Category:Industrial chemicals]] | ||
[[Category:Pollutants]] | [[Category:Pollutants]] | ||
{{stub}} | {{stub}} | ||
Latest revision as of 01:52, 24 February 2025
Xylene is an aromatic hydrocarbon widely used in the chemical industry and in medical technology. It is a colorless, sweet-smelling liquid or gas occurring naturally in petroleum, coal and wood tar, and is so named because it is found in crude wood spirit (Gr. ξύλον, wood).
Chemical Properties[edit]
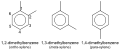
Xylene is a mixture of three different isomers: ortho-xylene, meta-xylene, and para-xylene. Each isomer is a benzene ring with two methyl groups attached at various positions. The chemical formula for xylene is C8H10.
Uses[edit]
Xylene is used as a solvent in the printing, rubber, and leather industries. It is also used as a cleaning agent, a thinner for paint, and in paints and varnishes. It is found in small amounts in airplane fuel and gasoline. Xylene is also used in the laboratory to make baths with dry ice to cool reaction vessels, and as a solvent to remove synthetic immersion oil from the microscope objective in light microscopy.
Health Effects[edit]
Exposure to xylene can occur via inhalation, ingestion, eye or skin contact. It affects the brain and can cause headaches, lack of muscle coordination, dizziness, confusion, and changes in one's sense of balance. Exposure of skin to xylene may cause skin irritation. Long-term exposure may lead to insomnia, stroke, or loss of appetite.
Safety[edit]
Xylene poses a risk of fire and explosion. It is harmful if inhaled, and irritating to the eyes, skin, and respiratory tract. It may cause effects on the central nervous system, resulting in impaired functions.
Xylene gallery[edit]
-
IUPAC cyclic