Van 't Hoff equation
Equation relating the change in equilibrium constant with temperature
The Van 't Hoff equation is an important equation in chemical thermodynamics that relates the change in the equilibrium constant of a chemical reaction to the change in temperature. It is named after the Dutch physical chemist Jacobus Henricus van 't Hoff, who was awarded the first Nobel Prize in Chemistry in 1901.
Equation[edit]
The Van 't Hoff equation is expressed as:
where:
- is the equilibrium constant of the reaction,
- is the temperature in Kelvins,
- is the standard enthalpy change of the reaction,
- is the universal gas constant.
This differential form of the Van 't Hoff equation can be integrated to give:
where and are the equilibrium constants at temperatures and , respectively.
Applications[edit]
The Van 't Hoff equation is used to estimate the effect of temperature on the position of equilibrium in chemical reactions. It is particularly useful in predicting whether a reaction will be more favorable at higher or lower temperatures.
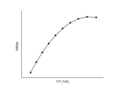
Endothermic Reactions[edit]
For an endothermic reaction, where , the equilibrium constant increases with an increase in temperature. This is illustrated in the following plot:

Exothermic Reactions[edit]
For an exothermic reaction, where , the equilibrium constant decreases with an increase in temperature. This behavior is shown in the plot below:

Van 't Hoff Plot[edit]
A Van 't Hoff plot is a graph of versus . The slope of the line is equal to , and the intercept can be used to determine the standard entropy change .

Mechanism Studies[edit]
Van 't Hoff plots are also used in reaction mechanism studies to determine the enthalpy and entropy changes associated with different steps in a reaction mechanism.

Temperature Dependence[edit]
The Van 't Hoff equation provides insight into the temperature dependence of reaction equilibria, which is crucial for understanding and optimizing chemical processes.

Related pages[edit]
References[edit]
- Atkins, P., & de Paula, J. (2006). Physical Chemistry. Oxford University Press.
- Laidler, K. J. (1987). Chemical Kinetics. Harper & Row.
Van 't Hoff equation[edit]
-
Endothermic Reaction van 't Hoff Plot
-
Exothermic Reaction van 't Hoff Plot
-
Van 't Hoff analysis
-
Van 't Hoff plot in Mechanism study
-
Temperature dependence van 't Hoff plot
Ad. Transform your life with W8MD's Budget GLP-1 injections from $49.99


W8MD offers a medical weight loss program to lose weight in Philadelphia. Our physician-supervised medical weight loss provides:
- Weight loss injections in NYC (generic and brand names):
- Zepbound / Mounjaro, Wegovy / Ozempic, Saxenda
- Most insurances accepted or discounted self-pay rates. We will obtain insurance prior authorizations if needed.
- Generic GLP1 weight loss injections from $49.99 for the starting dose of Semaglutide and $65.00 for Tirzepatide.
- Also offer prescription weight loss medications including Phentermine, Qsymia, Diethylpropion, Contrave etc.
NYC weight loss doctor appointmentsNYC weight loss doctor appointments
Start your NYC weight loss journey today at our NYC medical weight loss and Philadelphia medical weight loss clinics.
- Call 718-946-5500 to lose weight in NYC or for medical weight loss in Philadelphia 215-676-2334.
- Tags:NYC medical weight loss, Philadelphia lose weight Zepbound NYC, Budget GLP1 weight loss injections, Wegovy Philadelphia, Wegovy NYC, Philadelphia medical weight loss, Brookly weight loss and Wegovy NYC
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian