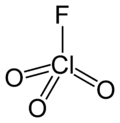
Perchloryl fluoride
Perchloryl fluoride is a chemical compound with the formula ClO_3F. It is a colorless gas at room temperature and is highly reactive, as well as toxic. Perchloryl fluoride is used primarily in the synthesis of organic compounds and as an oxidizing agent in rocket propellants. Due to its reactive nature, it is of significant interest in both industrial and academic settings for its potential applications and hazards.
Properties[edit]
Perchloryl fluoride is characterized by its strong oxidizing properties. It is capable of supporting combustion, even in the absence of atmospheric oxygen, which makes it particularly useful in closed-system applications such as rocket propulsion. The compound is stable under standard conditions but decomposes upon contact with water, releasing hydrochloric acid (HCl) and oxygen gas (O2), which can pose significant handling risks.
Synthesis[edit]
The synthesis of perchloryl fluoride typically involves the reaction of chlorine trifluoride (ClF3) with sodium perchlorate (NaClO4). This process requires careful control of reaction conditions to prevent uncontrolled decomposition or reaction, which could lead to explosive outcomes.
Applications[edit]
Organic Synthesis[edit]
In organic chemistry, perchloryl fluoride is used as a fluorinating agent, introducing fluorine atoms into organic molecules. This can significantly alter the physical and chemical properties of the compounds, making them more reactive or altering their biological activity.
Rocket Propellants[edit]
Due to its strong oxidizing properties, perchloryl fluoride is used in some rocket propellant formulations. It can significantly increase the efficiency of the propellant, providing higher thrust compared to traditional oxidizers.
Safety and Handling[edit]
Handling perchloryl fluoride requires strict safety measures due to its toxic and reactive nature. Exposure to the compound can lead to severe respiratory issues and chemical burns. In industrial settings, appropriate personal protective equipment (PPE) and engineering controls are essential to mitigate these risks.
Environmental Impact[edit]
The environmental impact of perchloryl fluoride is a concern due to its potential to decompose into chlorine and oxygen radicals, which can contribute to ozone layer depletion. As such, its use and disposal are regulated under various international environmental protection guidelines.
See Also[edit]
Perchloryl_fluoride[edit]
-
Perchloryl fluoride 2D structure
-
Perchloryl fluoride 3D van der Waals model
Ad. Transform your life with W8MD's Budget GLP-1 injections from $75


W8MD offers a medical weight loss program to lose weight in Philadelphia. Our physician-supervised medical weight loss provides:
- Weight loss injections in NYC (generic and brand names):
- Zepbound / Mounjaro, Wegovy / Ozempic, Saxenda
- Most insurances accepted or discounted self-pay rates. We will obtain insurance prior authorizations if needed.
- Generic GLP1 weight loss injections from $75 for the starting dose.
- Also offer prescription weight loss medications including Phentermine, Qsymia, Diethylpropion, Contrave etc.
NYC weight loss doctor appointmentsNYC weight loss doctor appointments
Start your NYC weight loss journey today at our NYC medical weight loss and Philadelphia medical weight loss clinics.
- Call 718-946-5500 to lose weight in NYC or for medical weight loss in Philadelphia 215-676-2334.
- Tags:NYC medical weight loss, Philadelphia lose weight Zepbound NYC, Budget GLP1 weight loss injections, Wegovy Philadelphia, Wegovy NYC, Philadelphia medical weight loss, Brookly weight loss and Wegovy NYC
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian