Favipiravir
Favipiravir, also known by its brand name Avigan, is an antiviral medication that has been used in the treatment of various viral infections, most notably influenza. Its mechanism of action involves inhibiting the RNA-dependent RNA polymerase (RdRp) of RNA viruses, thereby preventing viral replication. Favipiravir has garnered attention for its potential use in treating other viral infections, including Ebola virus disease and COVID-19.
Mechanism of Action[edit]
Favipiravir is a prodrug that, once ingested, is converted into its active form, favipiravir-RTP (ribonucleotide triphosphate). This active form directly inhibits the RdRp enzyme, which is crucial for the replication of RNA viruses. By blocking this enzyme, favipiravir prevents the virus from multiplying within the host's cells.
Clinical Uses[edit]
Originally developed for treating influenza, favipiravir has been explored for its efficacy against a broader range of RNA viruses. During the Ebola outbreak in 2014, favipiravir showed promise in reducing mortality rates among those infected. More recently, its potential effectiveness against the novel coronavirus (SARS-CoV-2), which causes COVID-19, has been under investigation. However, its use in COVID-19 has been met with mixed results, and further research is needed to fully understand its efficacy and safety profile in this context.
Side Effects[edit]
The use of favipiravir is associated with several side effects, including hyperuricemia, diarrhea, neutropenia, and liver enzyme elevations. Its safety in pregnant women has not been established, as animal studies have shown teratogenic effects. Therefore, favipiravir is contraindicated in pregnant women and in men who are not using effective contraception.
Regulatory Status[edit]
Favipiravir has been approved for use in several countries, including Japan and India, primarily for the treatment of influenza. Its approval and use for COVID-19 treatment vary by country, with some countries granting emergency use authorization while others await more conclusive evidence from clinical trials.
Research and Development[edit]
Ongoing research into favipiravir includes clinical trials aimed at evaluating its efficacy and safety in treating COVID-19 and other viral infections. The drug's ability to inhibit a wide range of RNA viruses makes it a subject of interest for future antiviral therapies.
Conclusion[edit]
Favipiravir represents a potentially valuable tool in the fight against viral diseases, thanks to its broad-spectrum antiviral activity. However, its use must be carefully weighed against its side effects, and its efficacy against specific viruses like SARS-CoV-2 requires further validation through clinical research.
-
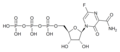
Structure of Favipiravir-RTP
-
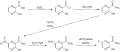
Synthesis of Favipiravir
-
Favipiravir Tablets 800 mg
-
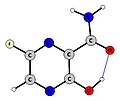
Avigan Enol Form
-
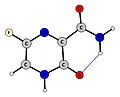
Avigan Keto Form
Ad. Transform your life with W8MD's Budget GLP-1 injections from $75


W8MD offers a medical weight loss program to lose weight in Philadelphia. Our physician-supervised medical weight loss provides:
- Weight loss injections in NYC (generic and brand names):
- Zepbound / Mounjaro, Wegovy / Ozempic, Saxenda
- Most insurances accepted or discounted self-pay rates. We will obtain insurance prior authorizations if needed.
- Generic GLP1 weight loss injections from $75 for the starting dose.
- Also offer prescription weight loss medications including Phentermine, Qsymia, Diethylpropion, Contrave etc.
NYC weight loss doctor appointmentsNYC weight loss doctor appointments
Start your NYC weight loss journey today at our NYC medical weight loss and Philadelphia medical weight loss clinics.
- Call 718-946-5500 to lose weight in NYC or for medical weight loss in Philadelphia 215-676-2334.
- Tags:NYC medical weight loss, Philadelphia lose weight Zepbound NYC, Budget GLP1 weight loss injections, Wegovy Philadelphia, Wegovy NYC, Philadelphia medical weight loss, Brookly weight loss and Wegovy NYC
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian