Ene reaction
Ene Reaction
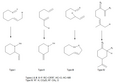
The Ene Reaction is a fundamental chemical reaction that involves the transfer of a hydrogen atom from one molecule (the ene) to an unsaturated species (the enophile) in the presence of a catalyst. This reaction is a key process in organic chemistry, particularly in the synthesis of complex natural products and the modification of unsaturated compounds. The Ene Reaction is characterized by its versatility and efficiency in forming carbon-carbon and carbon-heteroatom bonds, making it an essential tool in the arsenal of synthetic chemists.
Mechanism[edit]
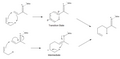
The Ene Reaction proceeds through a concerted mechanism, which involves the simultaneous formation and breaking of bonds. The reaction typically occurs through a six-membered transition state, where the hydrogen atom is transferred from the ene to the enophile, resulting in the formation of a new alkene and a new bond between the ene and enophile. The reaction can be catalyzed by both acids and bases, with the choice of catalyst affecting the reaction rate and selectivity.
Types of Ene Reactions[edit]
There are several variations of the Ene Reaction, each with its own specific substrates and conditions. These include:
- Thermal Ene Reactions: Occur without a catalyst at elevated temperatures. These reactions are typically less selective but can be useful for substrates that are stable to heat.
- Catalytic Ene Reactions: Involve the use of a catalyst, such as a Lewis acid or a Brønsted acid, to lower the activation energy and increase the reaction rate. Catalytic ene reactions are more selective and can be conducted at lower temperatures.
- Asymmetric Ene Reactions: A subset of catalytic ene reactions that employ chiral catalysts to produce enantiomerically enriched products. These reactions are important in the synthesis of chiral molecules.
Applications[edit]
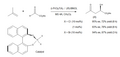
The Ene Reaction finds widespread application in organic synthesis, including the synthesis of natural products, pharmaceuticals, and polymers. Its ability to efficiently create complex molecules from simpler precursors has made it a valuable tool in the development of new drugs and materials.
Limitations[edit]
Despite its versatility, the Ene Reaction has some limitations. The reaction's efficiency and selectivity can be affected by the nature of the substrates and the reaction conditions. Additionally, some substrates may undergo competing reactions, such as polymerization or rearrangement, which can complicate the reaction outcome.
See Also[edit]
References[edit]
<references/>
-
Ene reaction
-
Ene reaction orbitals and transition state
-
Jatropha-5,12-diene carbonyl-ene reaction
-
Ene reaction
-
Ene reaction
-
Ene reaction
-
Ene reaction
-
Lewis acid-catalyzed ene reaction
-
Ene reaction
-
Ene reaction
-
Ene reaction
-
Ene reaction
Ad. Transform your life with W8MD's Budget GLP-1 injections from $75


W8MD offers a medical weight loss program to lose weight in Philadelphia. Our physician-supervised medical weight loss provides:
- Weight loss injections in NYC (generic and brand names):
- Zepbound / Mounjaro, Wegovy / Ozempic, Saxenda
- Most insurances accepted or discounted self-pay rates. We will obtain insurance prior authorizations if needed.
- Generic GLP1 weight loss injections from $75 for the starting dose.
- Also offer prescription weight loss medications including Phentermine, Qsymia, Diethylpropion, Contrave etc.
NYC weight loss doctor appointmentsNYC weight loss doctor appointments
Start your NYC weight loss journey today at our NYC medical weight loss and Philadelphia medical weight loss clinics.
- Call 718-946-5500 to lose weight in NYC or for medical weight loss in Philadelphia 215-676-2334.
- Tags:NYC medical weight loss, Philadelphia lose weight Zepbound NYC, Budget GLP1 weight loss injections, Wegovy Philadelphia, Wegovy NYC, Philadelphia medical weight loss, Brookly weight loss and Wegovy NYC
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian