Curtin–Hammett principle
Curtin–Hammett Principle
The Curtin–Hammett principle is a fundamental concept in the field of chemical kinetics and thermodynamics that explains the preference for the formation of one product over another in a chemical reaction, even when the reactants can proceed through two or more different transition states leading to different products. This principle is named after Donald J. Curtin and Louis P. Hammett, who independently formulated the concept in the 20th century. The principle has profound implications in the understanding of chemical equilibrium, reaction mechanisms, and the design of chemical reactions in both organic and inorganic chemistry.
Overview
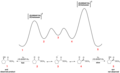
The Curtin–Hammett principle states that in a chemical equilibrium, the product distribution is determined not by the stability of the products themselves but by the difference in free energy of the transition states leading to those products. This means that the product ratio is dependent on the difference in activation energy between the competing reaction pathways, rather than the difference in energy between the final products.
Application
The principle is widely applied in organic synthesis to explain and predict the outcome of chemical reactions, especially when dealing with reactions that have competing pathways. It is particularly useful in understanding stereoselective and regioselective reactions, where the formation of one isomer or regioisomer is favored over another. The Curtin–Hammett principle helps chemists design reactions and select conditions that favor the formation of the desired product.
Key Concepts
- Transition State: The high-energy state through which reactants must pass to be converted into products. The energy required to reach the transition state from the reactants is the activation energy.
- Free Energy: A thermodynamic quantity that is a measure of the energy available to do work. The difference in free energy between the transition states determines the product distribution according to the Curtin–Hammett principle.
- Chemical Equilibrium: A state in a reversible chemical reaction where the rates of the forward and reverse reactions are equal, leading to constant concentrations of reactants and products.
Mathematical Description
The principle can be quantitatively described using the equation derived from the Gibbs free energy:
\[\Delta G^\ddagger = -RT \ln \left( \frac{k_1}{k_2} \right)\]
where \(\Delta G^\ddagger\) is the difference in Gibbs free energy between the two transition states, \(R\) is the gas constant, \(T\) is the temperature in Kelvin, and \(k_1\) and \(k_2\) are the rate constants for the competing reactions. This equation shows that the product ratio is determined by the difference in free energy of the transition states.
Limitations
While the Curtin–Hammett principle is widely applicable, it has limitations. It assumes that the interconversion between different reactant states is fast compared to the formation of the product, and that the reaction proceeds through well-defined transition states. In systems where these conditions are not met, the principle may not accurately predict the outcome of the reaction.
Conclusion
The Curtin–Hammett principle is a cornerstone of theoretical and applied chemistry, providing a framework for understanding and predicting the outcomes of chemical reactions based on the properties of transition states. Its application spans across various fields of chemistry, offering insights into reaction mechanisms and guiding the development of new synthetic methodologies.
Curtin–Hammett principle gallery
Transform your life with W8MD's budget GLP-1 injections from $125.
W8MD offers a medical weight loss program to lose weight in Philadelphia. Our physician-supervised medical weight loss provides:
- Most insurances accepted or discounted self-pay rates. We will obtain insurance prior authorizations if needed.
- Generic GLP1 weight loss injections from $125 for the starting dose.
- Also offer prescription weight loss medications including Phentermine, Qsymia, Diethylpropion, Contrave etc.
NYC weight loss doctor appointments
Start your NYC weight loss journey today at our NYC medical weight loss and Philadelphia medical weight loss clinics.
- Call 718-946-5500 to lose weight in NYC or for medical weight loss in Philadelphia 215-676-2334.
- Tags:NYC medical weight loss, Philadelphia lose weight Zepbound NYC, Budget GLP1 weight loss injections, Wegovy Philadelphia, Wegovy NYC, Philadelphia medical weight loss, Brookly weight loss and Wegovy NYC
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Contributors: Prab R. Tumpati, MD