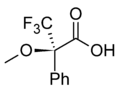
Chiral derivatizing agent
Chiral derivatizing agents (CDAs) are chemical compounds used in the analysis of chiral molecules. They play a crucial role in the field of chiral chromatography and NMR spectroscopy by enabling the differentiation between enantiomers of a chiral compound. These agents react with chiral substances to form diastereomers, which are distinguishable by their physical and chemical properties, facilitating the separation and identification of the enantiomers.
Overview[edit]
Chiral derivatizing agents are essential tools in analytical chemistry, especially in the pharmaceutical industry, where the chirality of a drug can affect its efficacy and safety. By forming diastereomers with different enantiomers, CDAs allow for the separation of these enantiomers using conventional analytical techniques, such as gas chromatography (GC), high-performance liquid chromatography (HPLC), or nuclear magnetic resonance (NMR) spectroscopy.
Mechanism[edit]
The mechanism of action of chiral derivatizing agents involves the formation of covalent bonds between the CDA and the chiral molecule. This reaction results in the creation of diastereomers, which are not mirror images of each other and therefore have different physical and chemical properties. These differences allow for the separation of the diastereomers using analytical techniques.
Types of Chiral Derivatizing Agents[edit]
Chiral derivatizing agents can be broadly classified based on their application in different analytical techniques:
For NMR Spectroscopy[edit]
In NMR spectroscopy, CDAs are used to induce shifts in the NMR signals of enantiomers, making it possible to distinguish between them. Examples include MOSHER'S ACID and its derivatives, which are widely used in the determination of enantiomeric purity and configuration.
For Chromatography[edit]
In chromatography, CDAs are used to modify chiral compounds, making them amenable to separation by GC or HPLC. Examples include derivatizing agents like (S)-Naproxen and (R)-Phenylethylamine, which are used to separate amino acids and other chiral compounds.
Applications[edit]
Chiral derivatizing agents find applications in various fields, including:
- Pharmaceutical analysis, where they are used to determine the enantiomeric purity of drugs.
- Biochemical research, for the study of biomolecules and their chiral properties.
- Environmental chemistry, in the analysis of chiral pollutants.
Challenges and Future Directions[edit]
While chiral derivatizing agents have significantly advanced the analysis of chiral molecules, there are challenges, such as the need for high-purity CDAs and the development of universal CDAs that can react with a wide range of chiral molecules. Ongoing research focuses on overcoming these challenges and improving the efficiency and applicability of CDAs in chiral analysis.
See Also[edit]
Ad. Transform your life with W8MD's Budget GLP-1 injections from $49.99


W8MD offers a medical weight loss program to lose weight in Philadelphia. Our physician-supervised medical weight loss provides:
- Weight loss injections in NYC (generic and brand names):
- Zepbound / Mounjaro, Wegovy / Ozempic, Saxenda
- Most insurances accepted or discounted self-pay rates. We will obtain insurance prior authorizations if needed.
- Generic GLP1 weight loss injections from $49.99 for the starting dose of Semaglutide and $65.00 for Tirzepatide.
- Also offer prescription weight loss medications including Phentermine, Qsymia, Diethylpropion, Contrave etc.
NYC weight loss doctor appointmentsNYC weight loss doctor appointments
Start your NYC weight loss journey today at our NYC medical weight loss and Philadelphia medical weight loss clinics.
- Call 718-946-5500 to lose weight in NYC or for medical weight loss in Philadelphia 215-676-2334.
- Tags:NYC medical weight loss, Philadelphia lose weight Zepbound NYC, Budget GLP1 weight loss injections, Wegovy Philadelphia, Wegovy NYC, Philadelphia medical weight loss, Brookly weight loss and Wegovy NYC
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian