Captodative effect
Captodative Effect
The captodative effect is a concept in organic chemistry that describes the stabilization of radical intermediates by substituents that are both electron-donating and electron-withdrawing. This phenomenon plays a crucial role in understanding the reactivity and selectivity of organic molecules undergoing radical reactions. The term "captodative" is derived from the words "capturing" and "donative," indicating the dual nature of the substituents involved in stabilizing the radical center.
Overview[edit]
In organic chemistry, radicals are highly reactive species with an unpaired electron. The stability of these radicals is influenced by the nature of the substituents attached to the radical center. Traditionally, it was believed that either electron-donating groups (EDGs) or electron-withdrawing groups (EWGs) could stabilize radicals. However, the captodative effect describes a scenario where the simultaneous presence of both types of groups leads to enhanced stabilization.
Mechanism[edit]
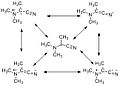
The captodative effect occurs due to the synergistic interaction between electron-donating and electron-withdrawing substituents attached to a radical center. EDGs donate electron density through sigma or pi bonds, while EWGs withdraw electron density through inductive or resonance effects. When both types of substituents are present, they create a delocalized electronic environment that stabilizes the radical. This stabilization is often greater than what would be expected from the sum of the individual effects of the EDG and EWG.
Implications[edit]
The captodative effect has significant implications for the selectivity and reactivity of radical reactions. It can influence the formation of radicals, their lifetimes, and their reaction pathways. Understanding this effect is crucial for designing synthetic strategies, especially in the synthesis of complex organic molecules where control over reactivity and selectivity is essential.
Examples[edit]
A classic example of the captodative effect can be seen in the stabilization of carbon-centered radicals. For instance, a radical bearing both an alkoxyl group (an EDG) and a cyano group (an EWG) exhibits enhanced stability due to the captodative effect. This stabilization can affect the rate and outcome of radical addition or abstraction reactions in organic synthesis.
Research and Applications[edit]
Research into the captodative effect continues to expand its applications in organic synthesis and materials science. By exploiting this effect, chemists can design molecules with specific reactivity patterns or create materials with unique properties. The captodative effect also has implications in understanding biological processes where radical intermediates play a role, such as in enzyme-catalyzed reactions.
Conclusion[edit]
The captodative effect is a fundamental concept in organic chemistry that enhances our understanding of radical stability and reactivity. By recognizing the role of both electron-donating and electron-withdrawing substituents, chemists can better predict and control the behavior of organic molecules in radical reactions.
-
Resonance contributors of 2-(dimethylamino)propanenitrile radical
-
Captodative effect
-
Thiophenoxide acrylonitrile transition states
-
2+2 cyclodimerisation and intramolecular cyclisation of captodative olefins
-
Comparison between Diels-Alder and captodative-enhanced Friedel-Crafts reaction
-
Solvent Affinity effects of substituents
-
Polar effect
Ad. Transform your life with W8MD's Budget GLP-1 injections from $75


W8MD offers a medical weight loss program to lose weight in Philadelphia. Our physician-supervised medical weight loss provides:
- Weight loss injections in NYC (generic and brand names):
- Zepbound / Mounjaro, Wegovy / Ozempic, Saxenda
- Most insurances accepted or discounted self-pay rates. We will obtain insurance prior authorizations if needed.
- Generic GLP1 weight loss injections from $75 for the starting dose.
- Also offer prescription weight loss medications including Phentermine, Qsymia, Diethylpropion, Contrave etc.
NYC weight loss doctor appointmentsNYC weight loss doctor appointments
Start your NYC weight loss journey today at our NYC medical weight loss and Philadelphia medical weight loss clinics.
- Call 718-946-5500 to lose weight in NYC or for medical weight loss in Philadelphia 215-676-2334.
- Tags:NYC medical weight loss, Philadelphia lose weight Zepbound NYC, Budget GLP1 weight loss injections, Wegovy Philadelphia, Wegovy NYC, Philadelphia medical weight loss, Brookly weight loss and Wegovy NYC
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian