Hydroxyestrone diacetate: Difference between revisions
CSV import |
No edit summary |
||
| Line 19: | Line 19: | ||
[[Category:Estrogen esters]] | [[Category:Estrogen esters]] | ||
[[Category:Synthetic estrogens]] | [[Category:Synthetic estrogens]] | ||
<gallery> | <gallery> | ||
File:Hydroxyestrone diacetate.svg|Hydroxyestrone diacetate | File:Hydroxyestrone diacetate.svg|Hydroxyestrone diacetate | ||
</gallery> | </gallery> | ||
Latest revision as of 00:01, 17 March 2025
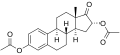
Hydroxyestrone diacetate is a synthetic, steroidal estrogen that was never marketed. It is an ester of hydroxyestrone, specifically, the 3,17β-diacetate ester.
Chemistry[edit]
Hydroxyestrone diacetate, also known as 16α-hydroxyestrone 3,17β-diacetate, is a synthetic estrane steroid and a derivative of estrone. It is more specifically a derivative of 16α-hydroxyestrone, which itself is a metabolite of estrone, and is a diester of hydroxyestrone formed from the substitution of the hydroxyl groups at the C3 and C17β positions with acetate esters.
Pharmacology[edit]
As an estrogen, hydroxyestrone diacetate has the ability to bind to and activate the estrogen receptor (ER). The affinity of hydroxyestrone diacetate for the ER and its estrogenic potency relative to estrone and other estrogens have not been studied. However, the 16α-hydroxylation of estrone has been found to significantly increase the estrogenic potency of the compound.
History[edit]
Hydroxyestrone diacetate was first synthesized in the late 1950s. It was never marketed, likely due to its poor oral bioavailability and the development of more effective synthetic estrogens.
See also[edit]
-
Hydroxyestrone diacetate