Gulose: Difference between revisions
CSV import |
CSV import |
||
| Line 40: | Line 40: | ||
[[Category:Aldoses]] | [[Category:Aldoses]] | ||
{{No image}} | {{No image}} | ||
== Gulose gallery == | |||
<gallery> | |||
File:Gulose.svg|Gulose | |||
</gallery> | |||
Revision as of 05:25, 3 March 2025
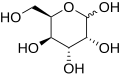
Gulose is a type of monosaccharide, specifically an aldose and a hexose sugar. It is one of the rare sugars that is not commonly found in nature. Gulose is an epimer of galactose, differing in the configuration around the fourth carbon atom.
Structure
Gulose has the chemical formula C₆H₁₂O₆. It exists in two enantiomeric forms: D-gulose and L-gulose. The D-form is the mirror image of the L-form. The structure of gulose can be represented in both its linear form and its cyclic form, which is more common in aqueous solutions.
Linear Form
In its linear form, gulose is an aldohexose, meaning it contains an aldehyde group (-CHO) at the first carbon. The linear structure can be represented as:
- HOCH₂-(CHOH)₄-CHO
Cyclic Form
In solution, gulose predominantly exists in a cyclic form, forming a six-membered ring known as a pyranose. The cyclic form is created when the hydroxyl group on the fifth carbon reacts with the aldehyde group, forming a hemiacetal linkage.
Stereochemistry
Gulose is an epimer of galactose, which means it differs from galactose only in the configuration around one specific carbon atom. In the case of gulose, this difference is at the C-4 position.
Occurrence
Gulose is not commonly found in nature. It is not a major component of any known biological systems, and it is not typically found in the human diet. However, it can be synthesized in the laboratory for research purposes.
Synthesis
Gulose can be synthesized from other sugars through a series of chemical reactions. One common method involves the epimerization of galactose. This process can be catalyzed by enzymes or chemical catalysts that facilitate the conversion of the hydroxyl group at the C-4 position.
Applications
While gulose itself is not widely used, its derivatives and analogs can be of interest in biochemical research. Researchers study rare sugars like gulose to understand their potential roles in biological systems and their possible applications in medicine and industry.
See Also
References
- John,
Biochemistry, Academic Press, 2020, ISBN 978-0-12-385245-8,
- Jones, Emily,
Rare Sugars: Chemistry and Applications, Journal of Carbohydrate Chemistry, 2019, Vol. 38(Issue: 4), pp. 123-145, DOI: 10.1080/07328303.2019.1581234,
Gulose gallery
-
Gulose