Schmidt reaction: Difference between revisions
CSV import |
CSV import |
||
| Line 24: | Line 24: | ||
[[Category:Name reactions]] | [[Category:Name reactions]] | ||
{{chemistry-stub}} | {{chemistry-stub}} | ||
== Schmidt reaction gallery == | |||
<gallery> | |||
File:Schmidt Reaktion Übersicht Carbonsäuren1.svg|Schmidt Reaction Overview Carboxylic Acids | |||
File:Schmidt Reaktion Übersicht Ketone1.svg|Schmidt Reaction Overview Ketones | |||
File:Schmidt reaction mechanism.svg|Schmidt Reaction Mechanism | |||
File:Schmidt reaction mechanism 2.svg|Schmidt Reaction Mechanism 2 | |||
File:BoyerReaction.png|Boyer Reaction | |||
File:SchmidtreactionApplication.png|Schmidt Reaction Application | |||
</gallery> | |||
Latest revision as of 05:16, 3 March 2025
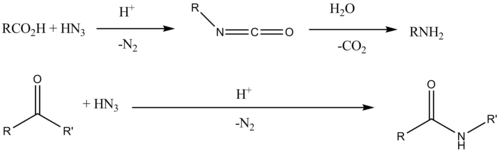
The Schmidt reaction is a chemical reaction that involves the conversion of ketones or aldehydes into amines or amides through the reaction with nitrogen sources, such as azides. This reaction is particularly significant in organic chemistry for the synthesis of complex nitrogen-containing compounds. The Schmidt reaction is named after the German chemist Karl Friedrich Schmidt, who first reported the reaction in 1924.
Mechanism[edit]
The Schmidt reaction proceeds through several key steps. Initially, the azide reacts with the carbonyl compound (ketone or aldehyde) to form an intermediate. This intermediate then undergoes a rearrangement, leading to the loss of nitrogen gas and the formation of a carbocation. The final step involves the capture of a nucleophile, which can be water in the case of amide formation or an amine for amine formation, resulting in the production of the desired product.
Variants[edit]
There are two main variants of the Schmidt reaction:
- The reaction of ketones or aldehydes with hydrazoic acid (HN3) in the presence of a strong acid, leading to the formation of amides or amines.
- The reaction of carboxylic acids with azides, which results in the formation of amides. This variant is particularly useful for the synthesis of lactams, cyclic amides that are core structures in many biologically active compounds.
Applications[edit]
The Schmidt reaction is widely used in the synthesis of pharmaceuticals, natural products, and other complex organic molecules. Its ability to introduce nitrogen atoms into organic frameworks efficiently makes it a valuable tool for the construction of a variety of nitrogen-containing compounds, including alkaloids, peptides, and heterocycles.
Limitations[edit]
Despite its utility, the Schmidt reaction has some limitations. The use of azides and strong acids can pose safety risks, and the reaction conditions may not be compatible with sensitive functional groups. Additionally, the reaction's selectivity and yield can be affected by the nature of the substrate and the specific reaction conditions employed.
See Also[edit]
Schmidt reaction gallery[edit]
-
Schmidt Reaction Overview Carboxylic Acids
-
Schmidt Reaction Overview Ketones
-
Schmidt Reaction Mechanism
-
Schmidt Reaction Mechanism 2
-
Boyer Reaction
-
Schmidt Reaction Application


