Scandium chloride: Difference between revisions
CSV import |
CSV import |
||
| Line 28: | Line 28: | ||
[[Category:Scandium compounds]] | [[Category:Scandium compounds]] | ||
{{Chem-stub}} | {{Chem-stub}} | ||
== Scandium chloride gallery == | |||
<gallery> | |||
File:Bismuth-triiodide-layer-3D-balls.png|Bismuth triiodide layer 3D balls | |||
File:ScCl3x6H2O.jpg|ScCl3x6H2O | |||
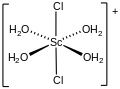
File:Trans-(Sc(aquo)4Cl2)+.svg|Trans-(Sc(aquo)4Cl2)+ | |||
</gallery> | |||
Latest revision as of 05:04, 3 March 2025
Scandium Chloride is a chemical compound with the formula ScCl3. It is a white, high-melting solid that is soluble in water and ethanol. Scandium chloride is used in various applications, including organic synthesis, the preparation of scandium metal, and in the field of materials science for the production of advanced materials.
Properties[edit]
Scandium chloride exists in several forms, including the anhydrous form (ScCl3) and hydrates such as ScCl3·6H2O. The anhydrous form is a white or slightly yellow solid with a high melting point, while the hydrates are more readily soluble in water.
Chemical[edit]
Scandium chloride reacts with water to form hydrated forms. In organic synthesis, it can act as a Lewis acid, facilitating various chemical reactions. It is also involved in the preparation of other scandium compounds.
Physical[edit]
The anhydrous form of scandium chloride has a high melting point and is relatively stable under normal conditions. It is soluble in water and ethanol, making it useful in various chemical processes.
Production[edit]
Scandium chloride is typically produced by the chlorination of scandium oxide (Sc2O3) with carbon tetrachloride (CCl4) or by reacting scandium metal with chlorine gas. The process yields anhydrous ScCl3, which can then be hydrated if necessary.
Applications[edit]
Scandium chloride is used in the synthesis of organic compounds as a Lewis acid. It is also a starting material for the production of high-purity scandium metal, which is used in advanced materials such as aerospace components and solid oxide fuel cells. Additionally, scandium chloride finds applications in the preparation of other scandium compounds and in materials science research.
Safety[edit]
Scandium chloride should be handled with care, as it is an irritant to the skin, eyes, and respiratory system. Appropriate safety measures, including the use of personal protective equipment, should be taken when handling this chemical.
See Also[edit]
Scandium chloride gallery[edit]
-
Bismuth triiodide layer 3D balls
-
ScCl3x6H2O
-
Trans-(Sc(aquo)4Cl2)+