Mauveine: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| Line 22: | Line 22: | ||
[[Category:Dyes]] | [[Category:Dyes]] | ||
{{Chemistry-stub}} | {{Chemistry-stub}} | ||
<gallery> | |||
File:Mauv2.jpg|Mauv2 | |||
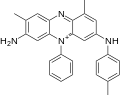
File:Mauveine a skeletal org.svg|Mauveine a skeletal org | |||
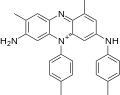
File:Mauveine b skeletal org.svg|Mauveine b skeletal org | |||
File:Mauveine b2 skeletal org.svg|Mauveine b2 skeletal org | |||
File:Mauveine c skeletal org.svg|Mauveine c skeletal org | |||
File:Charles Rees (in mauveine-dyed bowtie).jpg|Charles Rees (in mauveine-dyed bowtie) | |||
</gallery> | |||
Latest revision as of 05:04, 3 March 2025
Mauveine, also known as aniline purple and Perkin's mauve, is a synthetic organic compound of the formula C_27H_18N_4O_3. It was the first synthetic dye discovered in 1856 by English chemist William Henry Perkin, while he was attempting to synthesize quinine for the treatment of malaria. Mauveine marks the beginning of the synthetic dye and chemical industry, leading to the development of other synthetic dyes and the decline of natural dyes in the late 19th century.
History[edit]
The discovery of mauveine was a serendipitous event that occurred when Perkin, at the age of 18, was conducting experiments to synthesize quinine from aniline, a derivative of coal tar. Instead of producing quinine, he obtained a dark sludge. Upon further experimentation, he found that this sludge could be used to dye silk a beautiful shade of purple, which he named mauve. Recognizing the commercial potential of his discovery, Perkin patented the dye and opened a dye works in Greenford. The color mauve became extremely popular, especially after Queen Victoria wore a silk gown dyed with mauveine to the Royal Exhibition of 1862.
Chemical Properties[edit]
Mauveine is a mixture of several closely related compounds, with mauveine A and mauveine B being the most prominent. It is synthesized through the oxidation of a mixture of aniline and toluidine with potassium dichromate and sulfuric acid. The chemical structure of mauveine was not fully understood until the 1990s, when advanced analytical techniques such as mass spectrometry and nuclear magnetic resonance were applied.
Impact[edit]
The discovery of mauveine had a profound impact on the textile industry, as it allowed for the mass production of a wide range of synthetic dyes, which were more vibrant and colorfast than natural dyes. This innovation led to significant changes in fashion and fabric production, making colorful clothing more accessible to the general public. Furthermore, Perkin's work laid the foundation for the modern chemical industry, paving the way for the synthesis of other important compounds, including pharmaceuticals and explosives.
Legacy[edit]
In recognition of his contributions to chemistry and industry, Perkin was knighted in 1906. Today, mauveine is primarily of historical and academic interest, but its discovery is celebrated as a pivotal moment in the field of synthetic chemistry. The Perkin Medal, established in 1906, is awarded annually by the Society of Chemical Industry to a scientist residing in the United States for outstanding work in applied chemistry.
See Also[edit]
-
Mauv2
-
Mauveine a skeletal org
-
Mauveine b skeletal org
-
Mauveine b2 skeletal org
-
Mauveine c skeletal org
-
Charles Rees (in mauveine-dyed bowtie)