LY-334370: Difference between revisions
CSV import |
CSV import Tags: mobile edit mobile web edit |
||
| Line 29: | Line 29: | ||
{{Pharma-stub}} | {{Pharma-stub}} | ||
{{No image}} | {{No image}} | ||
== LY-334370 == | |||
<gallery> | |||
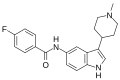
File:LY334370.svg|LY334370.svg | |||
File:LY-334370_3D_BS.png|LY-334370 3D BS.png | |||
</gallery> | |||
Revision as of 21:41, 23 February 2025
LY-334370 is a pharmaceutical drug that was developed by Eli Lilly and Company, a multinational pharmaceutical company. It is a selective 5-HT1F receptor agonist, and was primarily researched for the treatment of migraine.
History
LY-334370 was first synthesized by researchers at Eli Lilly and Company. The drug was developed as a part of the company's ongoing research into the therapeutic potential of 5-HT1F receptor agonists. The development of LY-334370 was based on the hypothesis that selective activation of 5-HT1F receptors could provide relief from migraine without the cardiovascular side effects associated with other serotonin receptor agonists.
Pharmacology
LY-334370 is a selective agonist for the 5-HT1F receptor, a type of serotonin receptor. Serotonin receptors are a group of G protein-coupled receptors (GPCRs) and ligand-gated ion channels found in the central nervous system and peripheral nervous system. The 5-HT1F receptor subtype is primarily found in the brain, and is thought to play a role in the pathophysiology of migraine.
Clinical Trials
In clinical trials, LY-334370 was found to be effective in the treatment of acute migraine. However, the development of the drug was discontinued for reasons that were not publicly disclosed. Despite the discontinuation of LY-334370, the results of the clinical trials provided valuable evidence for the potential of 5-HT1F receptor agonists in the treatment of migraine.
Legacy
The research into LY-334370 has had a significant impact on the field of migraine research. The positive results from the clinical trials of LY-334370 led to further research into 5-HT1F receptor agonists. This research eventually led to the development of Lasmiditan, another 5-HT1F receptor agonist developed by Eli Lilly and Company, which was approved by the Food and Drug Administration (FDA) for the treatment of acute migraine in 2019.
See Also
LY-334370
-
LY334370.svg
-
LY-334370 3D BS.png