LY-272,015: Difference between revisions
CSV import |
CSV import |
||
| Line 30: | Line 30: | ||
{{Pharma-stub}} | {{Pharma-stub}} | ||
{{No image}} | {{No image}} | ||
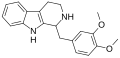
<gallery> | |||
File:LY-272,015 structure.svg|LY-272,015 | |||
</gallery> | |||
Revision as of 01:14, 20 February 2025
LY-272,015 is a pharmaceutical drug developed by Eli Lilly and Company. It is a selective serotonin reuptake inhibitor (SSRI) and was primarily researched for its potential use in the treatment of depression and anxiety disorders. However, the development of LY-272,015 was discontinued in the late 1990s due to unfavorable clinical trial results.
History
LY-272,015 was first synthesized by researchers at Eli Lilly and Company in the early 1990s. The drug was part of a larger effort by the company to develop new SSRIs with improved efficacy and fewer side effects than existing drugs like fluoxetine (Prozac).
Pharmacology
As an SSRI, LY-272,015 works by inhibiting the reuptake of serotonin in the brain, thereby increasing the amount of this neurotransmitter available in the synaptic cleft. This is believed to help alleviate the symptoms of depression and anxiety.
Clinical Trials
LY-272,015 underwent several phases of clinical trials in the mid to late 1990s. Initial results were promising, with the drug showing potential efficacy in treating depression and anxiety. However, later trials revealed a high incidence of side effects, including nausea, insomnia, and sexual dysfunction. These findings led Eli Lilly to discontinue the development of LY-272,015.
Discontinuation
The discontinuation of LY-272,015 was a significant setback for Eli Lilly, as the company had invested substantial resources in the drug's development. However, the experience with LY-272,015 helped inform the company's later efforts in the field of SSRIs, leading to the successful development and marketing of other drugs.
See Also
-
LY-272,015