Distillation: Difference between revisions
CSV import |
CSV import |
||
| Line 37: | Line 37: | ||
[[Category:Unit operations]] | [[Category:Unit operations]] | ||
{{stub}} | {{stub}} | ||
<gallery> | |||
File:Simple_distillation_apparatus.svg|Distillation | |||
File:Zosimos_distillation_equipment.jpg|Distillation | |||
File:Hieronymus_Brunschwig_Liber_de_arte_Distillandi_CHF_AQ13x3.jpg|Distillation | |||
File:My_retort.jpg|Distillation | |||
File:Distillation_by_Retort.png|Distillation | |||
File:UkrainianVodkaStill.jpg|Distillation | |||
File:Dorf_Lore_-_Schnaps-Destillation.jpg|Distillation | |||
File:BatchDistill.svg|Distillation | |||
File:Simple_distillation_apparatus.svg|Distillation | |||
File:Vacuum_distillation_of_DMSO_at_70C.jpg|Distillation | |||
File:Perkin_triangle_distillation_apparatus.svg|Distillation | |||
File:Short_path_distillation_apparatus.svg|Distillation | |||
</gallery> | |||
Revision as of 12:18, 18 February 2025
Distillation is a process of separating the component substances from a liquid mixture by selective evaporation and condensation. Distillation may result in essentially complete separation (nearly pure components), or it may be a partial separation that increases the concentration of selected components of the mixture.
History
The concept of distillation has been around since ancient times and is thought to have been first used by the ancient Egyptians. The process was later refined by the Greeks and Romans and has been used throughout history for a variety of purposes, from making perfumes to producing alcohol.
Process
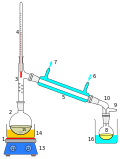
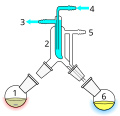
The process of distillation involves heating a liquid until it vaporizes, then cooling the vapor until it condenses back into a liquid. The condensed liquid is collected in a separate container. This process can be repeated multiple times to increase the purity of the desired component.
Types of Distillation
There are several types of distillation, including:
- Simple distillation: This is used when the boiling points of two liquids are significantly different from each other or to separate liquids from solids or nonvolatile components.
- Fractional distillation: This is used when the boiling points of the components are close to each other. It involves the use of a fractionating column.
- Steam distillation: This is used for substances that are heat sensitive. The substance is mixed with water and heated, the steam carries the volatile substances to a condenser where they are separated.
- Vacuum distillation: This is used for substances that have high boiling points. Lowering the pressure lowers the boiling points of the substances, allowing them to be distilled at lower temperatures.
Applications
Distillation has many applications, including:
- In the petroleum industry, where it is used in the refining of crude oil into its components, including gasoline, lubricating oils, and waxes.
- In the chemical industry, where it is used in the production of solvents, chemicals, and other products.
- In the food and beverage industry, where it is used in the production of alcoholic beverages, vinegar, and purified water.
- In the pharmaceutical industry, where it is used in the production of medicines and other products.