Cook–Heilbron thiazole synthesis: Difference between revisions
CSV import |
CSV import |
||
| Line 23: | Line 23: | ||
{{Chemistry-stub}} | {{Chemistry-stub}} | ||
== Cook–Heilbron thiazole synthesis == | |||
<gallery> | |||
File:Thiazole_priority_numbering.png|Thiazole priority numbering | |||
File:Cook-Heilbron_thiazole_synthesis_reaction_overview.png|Cook–Heilbron thiazole synthesis reaction overview | |||
File:Cook_heilbron_thiazole_synthesis_png.png|Cook–Heilbron thiazole synthesis | |||
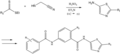
File:Example_of_an_Application_of_the_Cook-Heilbron_Thiazole_Synthesis.png|Example of an application of the Cook–Heilbron thiazole synthesis | |||
</gallery> | |||
Latest revision as of 05:06, 18 February 2025
Cook–Heilbron thiazole synthesis is a chemical reaction used in organic chemistry for the synthesis of thiazole derivatives. Thiazoles are heterocyclic compounds containing both sulfur and nitrogen in a five-membered ring. This synthesis method is named after the chemists Arthur H. Cook and Frederick G. Heilbron, who first reported it in the early 20th century. The Cook–Heilbron synthesis is notable for its ability to construct thiazole rings through the condensation of α-haloketones (or α-halo ketones) with thioamides.
Reaction Mechanism[edit]
The Cook–Heilbron thiazole synthesis involves several key steps. Initially, an α-haloketone reacts with a thioamide. This reaction leads to the formation of an intermediate that undergoes cyclization to form the thiazole ring. The process typically requires a base to facilitate the reaction. The overall mechanism highlights the importance of both the α-haloketone and thioamide as precursors in the synthesis of thiazoles.
Applications[edit]
Thiazoles are important in both pharmaceuticals and agrochemicals due to their biological activity. Compounds containing the thiazole ring have been found to exhibit a wide range of therapeutic properties, including antibacterial, antifungal, and anti-inflammatory activities. Therefore, the Cook–Heilbron thiazole synthesis is a valuable tool in the development of new drugs and agrochemical products.
Advantages and Limitations[edit]
One of the main advantages of the Cook–Heilbron synthesis is its straightforward approach to constructing thiazole rings, which are core structures in many biologically active compounds. However, like many chemical reactions, it has its limitations, including the need for specific starting materials (α-haloketones and thioamides) and conditions that may not be suitable for all types of substrates.
See Also[edit]
References[edit]
<references/>
Cook–Heilbron thiazole synthesis[edit]
-
Thiazole priority numbering
-
Cook–Heilbron thiazole synthesis reaction overview
-
Cook–Heilbron thiazole synthesis
-
Example of an application of the Cook–Heilbron thiazole synthesis