Eutectic system: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| Line 26: | Line 26: | ||
{{Chem-stub}} | {{Chem-stub}} | ||
<gallery> | |||
File:Eutectic_system_phase_diagram.svg|Eutectic system phase diagram | |||
File:Various_eutectic_structures.png|Various eutectic structures | |||
File:Phase_diagram_ethanol_water_s_l_en.svg|Phase diagram ethanol-water | |||
File:Iron_carbon_phase_diagram.svg|Iron-carbon phase diagram | |||
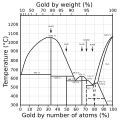
File:Phasendiagramm_Gold-Aluminium.svg|Gold-Aluminium phase diagram | |||
</gallery> | |||
Latest revision as of 05:01, 18 February 2025
Eutectic system refers to a homogeneous mixture of substances that melts or solidifies at a single temperature that is lower than the melting points of the individual substances. This unique characteristic makes eutectic systems important in various fields, including pharmaceutics, materials science, and metallurgy. The term "eutectic" is derived from the Greek words "eu" meaning good or well and "tēxis" meaning melting; thus, eutectic translates to "easily melted."
Composition and Characteristics[edit]
A eutectic system consists of two or more components that, when mixed in a specific ratio, form a eutectic mixture. This mixture has a distinct melting point, known as the eutectic point, which is the lowest possible melting point across all mixing ratios of the components. At the eutectic point, the solid phases of the components coexist in equilibrium with the liquid phase. The composition of this mixture at the eutectic point is called the eutectic composition.
Eutectic Reaction[edit]
The eutectic reaction is described by the formula: \[L \rightarrow \alpha + \beta\] where \(L\) represents the liquid phase, and \(\alpha\) and \(\beta\) represent the different solid phases that coexist at the eutectic point. This reaction is reversible, meaning that upon cooling, a liquid mixture of the eutectic composition solidifies into two separate phases simultaneously at the eutectic temperature.
Applications[edit]
Eutectic systems have a wide range of applications due to their unique melting and solidification properties. In pharmaceutics, eutectic mixtures are used to lower the melting point of substances, thereby enhancing their solubility and absorption. In materials science, eutectic alloys, such as solder, are valued for their low melting points and good mechanical properties. In metallurgy, eutectic compositions are exploited in casting, as they allow for the production of materials with fine microstructures and excellent wear resistance.
Examples[edit]
One of the most well-known eutectic systems is the lead-tin system, widely used in solder. The eutectic composition for lead and tin is approximately 63% tin and 37% lead, melting at 183°C, which is lower than the melting points of both pure lead (327°C) and pure tin (232°C). Another example is the eutectic mixture of menthol and camphor in topical analgesics, which enhances the delivery of active ingredients through the skin.
Phase Diagram[edit]
The behavior of eutectic systems can be represented by a phase diagram, which plots temperature against the composition of the mixture. The eutectic point is indicated on the diagram where the liquid phase line meets the solidus line. This point represents the lowest temperature at which the liquid phase can exist.
Conclusion[edit]
Eutectic systems play a crucial role in various scientific and industrial fields due to their unique properties. Understanding these systems allows for the development of materials and products with specific melting and solidification characteristics, tailored for particular applications.
-
Eutectic system phase diagram
-
Various eutectic structures
-
Phase diagram ethanol-water
-
Iron-carbon phase diagram
-
Gold-Aluminium phase diagram