Enthalpy: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| Line 33: | Line 33: | ||
{{stub}} | {{stub}} | ||
{{dictionary-stub1}} | {{dictionary-stub1}} | ||
<gallery> | |||
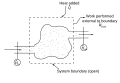
File:First_law_open_system.svg|First law of thermodynamics for an open system | |||
File:Ts_diagram_of_N2_02.jpg|T-s diagram of nitrogen | |||
File:Schematic_of_throttling.png|Schematic of a throttling process | |||
File:Schematic_of_compressor.png|Schematic of a compressor | |||
</gallery> | |||
Latest revision as of 04:53, 18 February 2025
Enthalpy is a concept used in thermodynamics to understand the total heat content of a system. It is a state function that depends only on the prevailing equilibrium state identified by the system's internal energy, pressure, and volume. It is an extensive quantity.
Definition[edit]
The enthalpy of a system is defined as the sum of its internal energy U and the product of its pressure P and volume V. The equation for enthalpy H is:
- H = U + PV
Understanding Enthalpy[edit]
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.
Enthalpy Change[edit]
The change in enthalpy (ΔH) of a reaction is a useful quantity, as it is directly measurable. It is the energy transferred from the system to its surroundings, or vice versa, in a process that takes place at constant pressure.
Enthalpy in Chemical Reactions[edit]
In chemical reactions, the enthalpy of reaction indicates whether a reaction is exothermic (releases heat, ΔH < 0) or endothermic (absorbs heat, ΔH > 0).