Pentane: Difference between revisions
CSV import |
CSV import |
||
| Line 26: | Line 26: | ||
[[Category:Solvents]] | [[Category:Solvents]] | ||
{{Chem-stub}} | {{Chem-stub}} | ||
<gallery> | |||
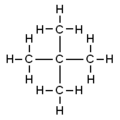
File:N-Pentan.png|n-Pentane | |||
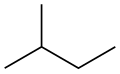
File:Isopentane.PNG|Isopentane | |||
File:Neopentane.PNG|Neopentane | |||
File:Pentane-2D-Skeletal.svg|n-Pentane 2D Skeletal | |||
File:Isopentane-2D-skeletal.svg|Isopentane 2D Skeletal | |||
File:Neopentane-2D-skeletal.png|Neopentane 2D Skeletal | |||
</gallery> | |||
Latest revision as of 04:31, 18 February 2025
Pentane is a type of hydrocarbon that belongs to the alkane series. It is a colorless liquid at room temperature and is primarily used as a solvent. It is also used as a fuel in some industrial applications.
Chemical Structure[edit]
Pentane has the chemical formula C5H12. It consists of five carbon atoms and twelve hydrogen atoms. The carbon atoms are arranged in a straight chain, making pentane a straight-chain alkane.
Physical Properties[edit]
Pentane is a colorless liquid at room temperature with a characteristic odor. It has a boiling point of 36.1 degrees Celsius and a melting point of -129.8 degrees Celsius. It is less dense than water and is insoluble in water but soluble in ethanol and ether.
Chemical Properties[edit]
Pentane is highly flammable and can form explosive mixtures with air. It undergoes combustion to produce carbon dioxide and water. It can also react with halogens in a halogenation reaction to form halogenated compounds.
Uses[edit]
Pentane is primarily used as a solvent in the laboratory. It is also used as a fuel in some industrial applications. In addition, it is used in the production of polystyrene foam and other types of foam.
Safety[edit]
Pentane is highly flammable and should be handled with care. It can cause skin and eye irritation and may be harmful if inhaled or swallowed.
See Also[edit]
-
n-Pentane
-
Isopentane
-
Neopentane
-
n-Pentane 2D Skeletal
-
Isopentane 2D Skeletal
-
Neopentane 2D Skeletal