Acifluorfen: Difference between revisions
CSV import |
CSV import |
||
| Line 37: | Line 37: | ||
[[Category:Agricultural chemicals]] | [[Category:Agricultural chemicals]] | ||
[[Category:Diphenyl ethers]] | [[Category:Diphenyl ethers]] | ||
<gallery> | |||
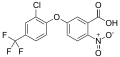
File:Acifluorfen_structure.svg|Chemical structure of Acifluorfen | |||
File:Acifluorfen_molecule_spacefill.png|Space-filling model of Acifluorfen molecule | |||
File:Acifluorfen_synthesis.svg|Synthesis pathway of Acifluorfen | |||
</gallery> | |||
Latest revision as of 01:44, 18 February 2025
A herbicide used in agriculture
| Chemical Compound | |
|---|---|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider ID | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Properties | |
| Chemical Formula | |
| Molar Mass | |
| Appearance | |
| Density | |
| Melting Point | |
| Boiling Point | |
| Hazards | |
| GHS Pictograms | [[File:|50px]] |
| GHS Signal Word | |
| GHS Hazard Statements | |
| NFPA 704 | [[File:|50px]] |
| References | |
Acifluorfen is a herbicide used primarily in agriculture to control broadleaf weeds and grasses. It is a member of the diphenyl ether class of herbicides and is known for its effectiveness in post-emergence weed control.
Chemical properties[edit]
Acifluorfen is a diphenyl ether derivative with the chemical formula C14H7ClF3NO5. It is characterized by its ability to inhibit the protoporphyrinogen oxidase enzyme, which is crucial in the chlorophyll biosynthesis pathway. This inhibition leads to the accumulation of protoporphyrin IX, causing cell membrane disruption and ultimately plant death.
Mode of action[edit]
Acifluorfen acts by inhibiting the enzyme protoporphyrinogen oxidase (PPO), which is involved in the biosynthesis of chlorophyll. The inhibition of PPO leads to the accumulation of protoporphyrin IX, a photodynamic compound that, in the presence of light, generates reactive oxygen species. These reactive oxygen species cause lipid peroxidation, leading to the destruction of cell membranes and plant tissues.
Applications[edit]
Acifluorfen is used in various crops such as soybeans, peanuts, and rice. It is applied as a post-emergence herbicide, meaning it is used after the weeds have emerged from the soil. Its effectiveness is enhanced by the presence of sunlight, which activates the photodynamic process leading to weed control.
Safety and environmental impact[edit]
While acifluorfen is effective in controlling weeds, it is important to consider its environmental impact and safety profile. It is classified as a restricted use pesticide in some regions due to its potential to cause harm to non-target plants and aquatic organisms. Proper handling and application techniques are essential to minimize its impact on the environment.
Synthesis[edit]
The synthesis of acifluorfen involves several chemical reactions, starting from basic organic compounds. The process typically includes the formation of the diphenyl ether backbone, followed by the introduction of the nitro, chloro, and trifluoromethyl groups. The detailed synthesis pathway is illustrated in the accompanying diagram.

Related pages[edit]
-
Chemical structure of Acifluorfen
-
Space-filling model of Acifluorfen molecule
-
Synthesis pathway of Acifluorfen