3-Hydroxypicolinic acid: Difference between revisions
CSV import |
CSV import |
||
| Line 35: | Line 35: | ||
[[Category:Pyridines]] | [[Category:Pyridines]] | ||
[[Category:Carboxylic acids]] | [[Category:Carboxylic acids]] | ||
== 3-Hydroxypicolinic_acid == | |||
<gallery> | |||
File:3_hydroxypicolinic_acid.svg|3-Hydroxypicolinic acid structure | |||
File:3-Hydroxypicolinic-acid-3D-balls.png|3-Hydroxypicolinic acid 3D model | |||
</gallery> | |||
Latest revision as of 01:32, 18 February 2025
3-Hydroxypicolinic acid[edit]


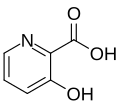
3-Hydroxypicolinic acid is an organic compound with the chemical formula C6H5NO3. It is a derivative of picolinic acid, which is a pyridine carboxylic acid. The compound is characterized by the presence of a hydroxyl group at the third position of the pyridine ring.
Structure and Properties[edit]
3-Hydroxypicolinic acid consists of a pyridine ring, which is a six-membered ring containing five carbon atoms and one nitrogen atom. The hydroxyl group (-OH) is attached to the third carbon of the pyridine ring, making it a hydroxylated derivative of picolinic acid. This structural modification imparts unique chemical properties to the compound, including its ability to act as a chelating agent.
Synthesis[edit]
The synthesis of 3-Hydroxypicolinic acid can be achieved through various chemical reactions, often involving the hydroxylation of picolinic acid. The process typically requires specific reagents and conditions to ensure the selective addition of the hydroxyl group at the desired position on the pyridine ring.
Applications[edit]
3-Hydroxypicolinic acid is used in various scientific and industrial applications. It is commonly employed as a chelating agent due to its ability to form stable complexes with metal ions. This property is particularly useful in analytical chemistry and biochemistry for the detection and quantification of metal ions in samples.
Biological Significance[edit]
In biological systems, 3-Hydroxypicolinic acid can play a role in metal ion homeostasis. Its chelating ability allows it to bind metal ions, which can be crucial in regulating their availability and activity within cells. This function is important in various physiological processes, including enzyme activity and cellular metabolism.
Related Compounds[edit]
3-Hydroxypicolinic acid is related to other pyridine carboxylic acids, such as picolinic acid and nicotinic acid. These compounds share a similar pyridine ring structure but differ in the functional groups attached to the ring, which influence their chemical and biological properties.
Related pages[edit]
3-Hydroxypicolinic_acid[edit]
-
3-Hydroxypicolinic acid structure
-
3-Hydroxypicolinic acid 3D model