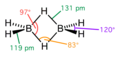Three-center two-electron bond: Difference between revisions
CSV import |
CSV import |
||
| Line 36: | Line 36: | ||
{{Chemistry-stub}} | {{Chemistry-stub}} | ||
<gallery> | |||
File:Diborane_resonance.svg|Diborane resonance structures | |||
File:Diborane-2D.png|Diborane 2D structure | |||
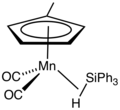
File:Cp'Mn(CO)2(HSiPh3).png|Three-center two-electron bond | |||
</gallery> | |||
Latest revision as of 01:20, 18 February 2025
Three-center two-electron bond (3c-2e) is a type of chemical bond in which three atoms share two electrons. This bonding situation is somewhat less common than the more familiar two-center two-electron (2c-2e) bond found in, for example, a covalent bond between two hydrogen atoms. The 3c-2e bond is an important concept in the chemistry of electron-deficient compounds, such as those found in boranes and certain organometallic compounds.
Overview[edit]
In a three-center two-electron bond, two electrons are shared by three atoms, rather than the typical scenario where two electrons are shared by two atoms. This type of bonding is crucial for stabilizing certain molecular structures that cannot be adequately described by conventional Lewis structures. It is most commonly observed in compounds of boron and hydrogen, such as in diborane (B2H6), where two hydrogen atoms bridge between two boron atoms, forming a bond that is delocalized over the three atoms.
Formation[edit]
The formation of a 3c-2e bond can be understood through molecular orbital theory. In this framework, the overlapping of atomic orbitals from the three involved atoms results in the formation of molecular orbitals that can accommodate two electrons. These electrons are then delocalized over the three atoms, leading to a bonding interaction that contributes to the stability of the molecule.
Characteristics[edit]
Three-center two-electron bonds have several distinctive characteristics:
- They often occur in molecules where conventional bonding theories cannot adequately explain the observed molecular structures.
- The bond strength of a 3c-2e bond is generally weaker than that of a conventional 2c-2e bond.
- Electron density in a 3c-2e bond is more delocalized compared to a 2c-2e bond.
Examples[edit]
- Diborane (B2H6): Perhaps the most well-known example of a molecule featuring 3c-2e bonds. In diborane, two hydrogen atoms bridge between two boron atoms.
- Borohydrides: Compounds such as sodium borohydride (NaBH4) contain boron-hydrogen 3c-2e bonds and are widely used in organic synthesis and as reducing agents.
- Organometallic compounds: Certain organometallic complexes feature 3c-2e bonding, particularly those involving electron-deficient metal centers.
Significance[edit]
The concept of three-center two-electron bonds is significant in several areas of chemistry:
- It provides a more accurate description of the bonding in electron-deficient compounds.
- It helps in understanding the reactivity and stability of compounds that cannot be explained by traditional bonding models.
- It has applications in the synthesis and design of new materials and catalysts, particularly in the field of organometallic chemistry.
See also[edit]
-
Diborane resonance structures
-
Diborane 2D structure
-
Three-center two-electron bond