Isethionic acid: Difference between revisions
CSV import |
CSV import |
||
| Line 32: | Line 32: | ||
[[Category:Articles with chemical identifiers]] | [[Category:Articles with chemical identifiers]] | ||
[[Category:Sulfonic acids]] | [[Category:Sulfonic acids]] | ||
== Isethionic_acid == | |||
<gallery> | |||
File:Isethionic-acid-2D-skeletal.png|2D skeletal structure of Isethionic acid | |||
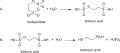
File:Isethionic_acid_2-step.svg|2-step synthesis of Isethionic acid | |||
File:Sodium_isethionate_EO.svg|Sodium isethionate structure | |||
</gallery> | |||
Latest revision as of 01:09, 18 February 2025
Isethionic Acid is an organic compound with the chemical formula C2H6O4S, specifically 2-hydroxyethanesulfonic acid. It is a colorless, water-soluble solid that plays a significant role in biochemistry, particularly in the metabolism of certain marine organisms. Isethionic acid is also of interest in various industrial applications due to its properties as a mild surfactant and its use in the synthesis of more complex chemical compounds.
Properties and Structure[edit]
Isethionic acid consists of a two-carbon chain (ethane) where one carbon is bonded to a hydroxyl group (-OH) and the other to a sulfonic acid group (-SO3H), making it a sulfonic acid derivative. This structure contributes to its high solubility in water and its hygroscopic nature. The presence of both the hydroxyl and sulfonic acid functional groups allows it to participate in a wide range of chemical reactions, making it a versatile compound in organic synthesis.
Biosynthesis and Biological Role[edit]
In nature, isethionic acid is produced by some marine organisms, such as algae and invertebrates, as a response to osmotic stress. It acts as an osmolyte, helping these organisms to maintain cell volume and fluid balance in the highly saline marine environment. The biosynthesis of isethionic acid in these organisms typically involves the direct sulfonation of ethanolamine, a process that is catalyzed by specific enzymes.
Industrial Applications[edit]
Isethionic acid and its derivatives find applications in various industries. Due to its mild surfactant properties, it is used in the formulation of personal care products such as shampoos and body washes, where it helps to moderate the action of stronger detergents and reduce skin irritation. Additionally, it serves as a starting material in the synthesis of more complex organic molecules, including pharmaceuticals and dyes.
Environmental Considerations[edit]
While isethionic acid is considered to be of low toxicity, its environmental impact, particularly in aquatic ecosystems, is an area of ongoing research. As with many chemical compounds, the potential effects of its widespread use and eventual release into the environment warrant careful consideration and study.
See Also[edit]
References[edit]
<references/>
Isethionic_acid[edit]
-
2D skeletal structure of Isethionic acid
-
2-step synthesis of Isethionic acid
-
Sodium isethionate structure