3-Indolepropionic acid: Difference between revisions
CSV import |
CSV import |
||
| Line 30: | Line 30: | ||
[[Category:Antioxidants]] | [[Category:Antioxidants]] | ||
[[Category:Human metabolites]] | [[Category:Human metabolites]] | ||
<gallery> | |||
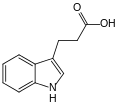
File:3-Indolepropionic_acid_skeletal.svg|Skeletal structure of 3-Indolepropionic acid | |||
</gallery> | |||
Latest revision as of 22:09, 16 February 2025
A naturally occurring indole-3-propionic acid
Overview[edit]
3-Indolepropionic acid (IPA) is a naturally occurring indole-propionic acid compound. It is a type of phytoalexin, which is a substance produced by plants that provides resistance against pathogens. IPA is also found in the human body, where it is produced by the gut microbiota.
Chemical Structure[edit]
The chemical structure of 3-Indolepropionic acid consists of an indole ring attached to a propionic acid moiety. The indole ring is a bicyclic structure composed of a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring. The propionic acid group is a three-carbon chain with a carboxylic acid functional group.

Biological Role[edit]
3-Indolepropionic acid is known for its antioxidant properties. It is considered a potent scavenger of hydroxyl radicals, which are highly reactive species that can cause oxidative damage to cells and tissues. The presence of IPA in the human body is primarily attributed to the activity of certain gut bacteria, such as Clostridium sporogenes, which convert dietary tryptophan into IPA.
Potential Health Benefits[edit]
Research suggests that 3-Indolepropionic acid may have several health benefits due to its antioxidant activity. It has been studied for its potential neuroprotective effects, particularly in the context of Alzheimer's disease and other neurodegenerative conditions. IPA may help in reducing oxidative stress and inflammation, which are key factors in the progression of these diseases.
Synthesis and Metabolism[edit]
In the human body, 3-Indolepropionic acid is synthesized from tryptophan, an essential amino acid, through the action of gut microbiota. The metabolic pathway involves the conversion of tryptophan to indole, which is then transformed into IPA. This process highlights the important role of the gut microbiome in modulating the levels of bioactive compounds in the body.
Related Pages[edit]
-
Skeletal structure of 3-Indolepropionic acid