Gas constant: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| Line 64: | Line 64: | ||
{{physics-stub}} | {{physics-stub}} | ||
<gallery> | |||
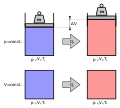
File:Heating-gas-at-constant-pressure-and-constant-volume.svg|Heating gas at constant pressure and constant volume | |||
</gallery> | |||
Latest revision as of 22:06, 16 February 2025
Physical constant in thermodynamics
The gas constant (also known as the universal gas constant, molar gas constant, or simply R) is a fundamental physical constant that appears in many fundamental equations in the physical sciences, such as the ideal gas law and the Nernst equation. It is equivalent to the Boltzmann constant, but expressed in units of energy per temperature increment per mole, i.e., the product of the Boltzmann constant and the Avogadro constant.
Definition[edit]
The gas constant is defined as:
- R = 8.314462618 J⋅K⁻¹⋅mol⁻¹
Ideal Gas Law[edit]
The gas constant is a key component of the ideal gas law, which is expressed as:
- PV = nRT
where:
- P is the pressure of the gas,
- V is the volume of the gas,
- n is the amount of substance of the gas (in moles),
- R is the gas constant,
- T is the temperature of the gas (in kelvins).
Relation to Other Constants[edit]
The gas constant can be related to other fundamental constants:
- R = N_A * k_B
where:
- N_A is the Avogadro constant,
- k_B is the Boltzmann constant.
Applications[edit]
The gas constant is used in various equations and applications in thermodynamics, physical chemistry, and statistical mechanics. Some of these include:
- The Nernst equation in electrochemistry,
- The Clausius–Clapeyron relation in phase transitions,
- The Arrhenius equation in chemical kinetics.
Units[edit]
The gas constant has different values depending on the units used. The most common value is:
- R = 8.314462618 J⋅K⁻¹⋅mol⁻¹
Other common units include:
- 0.0821 L⋅atm⋅K⁻¹⋅mol⁻¹
- 1.987 cal⋅K⁻¹⋅mol⁻¹
See Also[edit]
- Boltzmann constant
- Avogadro constant
- Ideal gas law
- Thermodynamics
- Physical chemistry
- Statistical mechanics
References[edit]
<references group="" responsive="1"></references>
-
Heating gas at constant pressure and constant volume