Atomic physics: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| Line 1: | Line 1: | ||
{{Short description|Overview of atomic physics and the Bohr model}} | |||
== Atomic Physics == | |||
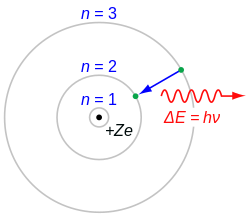
[[File:Bohr_atom_model.svg|thumb|right|200px|Diagram of the Bohr model of the atom]] | |||
'''Atomic physics''' is the field of [[physics]] that studies [[atoms]] as an isolated system of [[electrons]] and an atomic nucleus. It is primarily concerned with the arrangement of electrons around the nucleus and the processes by which these arrangements change. This field is fundamental to our understanding of the [[quantum mechanics|quantum mechanical]] nature of matter. | |||
== | === Historical Development === | ||
The study of atomic physics began with the development of the [[atomic theory]] in the early 19th century. The [[Bohr model]], introduced by [[Niels Bohr]] in 1913, was a major advancement in the field. It provided a new understanding of atomic structure and electron behavior. | |||
The | === The Bohr Model === | ||
The [[Bohr model]] is a [[quantum physics|quantum mechanical]] model of the atom that describes electrons orbiting the nucleus in discrete [[energy levels]]. According to this model, electrons can only occupy certain allowed orbits, and they emit or absorb [[photons]] when they transition between these orbits. | |||
== | ==== Key Features of the Bohr Model ==== | ||
* '''Quantized Orbits''': Electrons move in fixed orbits with quantized angular momentum. | |||
* '''Energy Levels''': Each orbit corresponds to a specific energy level. | |||
* '''Emission and Absorption''': Electrons emit or absorb energy in the form of photons when they jump between orbits. | |||
=== | === Limitations of the Bohr Model === | ||
While the Bohr model was successful in explaining the [[hydrogen atom]]'s spectral lines, it could not accurately predict the spectra of more complex atoms. This led to the development of more sophisticated models, such as the [[quantum mechanical model]] of the atom. | |||
== Applications of Atomic Physics == | |||
Atomic physics has numerous applications in various fields, including: | |||
== | * '''[[Spectroscopy]]''': The study of the interaction between matter and electromagnetic radiation. | ||
* '''[[Quantum computing]]''': Utilizing quantum states of atoms for computation. | |||
* '''[[Medical imaging]]''': Techniques such as [[MRI]] and [[CT scans]] rely on principles of atomic physics. | |||
== Related Pages == | |||
* [[Quantum mechanics]] | * [[Quantum mechanics]] | ||
* [[ | * [[Niels Bohr]] | ||
* [[ | * [[Spectroscopy]] | ||
* [[ | * [[Hydrogen atom]] | ||
[[Category:Atomic physics]] | [[Category:Atomic physics]] | ||
Latest revision as of 11:32, 15 February 2025
Overview of atomic physics and the Bohr model
Atomic Physics[edit]
Atomic physics is the field of physics that studies atoms as an isolated system of electrons and an atomic nucleus. It is primarily concerned with the arrangement of electrons around the nucleus and the processes by which these arrangements change. This field is fundamental to our understanding of the quantum mechanical nature of matter.
Historical Development[edit]
The study of atomic physics began with the development of the atomic theory in the early 19th century. The Bohr model, introduced by Niels Bohr in 1913, was a major advancement in the field. It provided a new understanding of atomic structure and electron behavior.
The Bohr Model[edit]
The Bohr model is a quantum mechanical model of the atom that describes electrons orbiting the nucleus in discrete energy levels. According to this model, electrons can only occupy certain allowed orbits, and they emit or absorb photons when they transition between these orbits.
Key Features of the Bohr Model[edit]
- Quantized Orbits: Electrons move in fixed orbits with quantized angular momentum.
- Energy Levels: Each orbit corresponds to a specific energy level.
- Emission and Absorption: Electrons emit or absorb energy in the form of photons when they jump between orbits.
Limitations of the Bohr Model[edit]
While the Bohr model was successful in explaining the hydrogen atom's spectral lines, it could not accurately predict the spectra of more complex atoms. This led to the development of more sophisticated models, such as the quantum mechanical model of the atom.
Applications of Atomic Physics[edit]
Atomic physics has numerous applications in various fields, including:
- Spectroscopy: The study of the interaction between matter and electromagnetic radiation.
- Quantum computing: Utilizing quantum states of atoms for computation.
- Medical imaging: Techniques such as MRI and CT scans rely on principles of atomic physics.